By Sarah C.P. Williams
November/December 2015
- Peptides that affect how cells function-known as “biopeptides”-are an important subject of study within the field of proteomics.
- Many biopeptides have an effect on metabolism or how the body digests food, whereas others have functional properties that are useful in cosmetics.
- This article will get you up to speed on biopeptides, which are likely to become more plentiful in food and medicine as scientists discover how to better isolate, study, and produce them.
In every cell of the human body, millions of proteins are buzzing with activity. Some are massive pieces of machinery, relatively speaking, that direct chemical reactions; others compose the scaffolding that gives cells their shape; and some act as vehicles to carry products and messages throughout the body. There’s no doubting their importance. But there is another class of molecule functioning in the shadow of these behemoths: peptides. The little siblings of proteins, peptides are smaller molecules composed of the same building blocks.
Despite their diminutive size-and often because of it-peptides have emerged as increasingly important biological entities capable of treating diseases, reducing inflammation, making foods more nutritious, killing microbes, and reversing aging. As scientists study the oversized properties of petite peptides, they are beginning to discover how to better isolate, study, and produce them. It is just the beginning for bioactive peptides.
Biopeptide Basics
Proteins and peptides alike are made of strings of proteinogenic, or standard, amino acids-22 organic chemical building blocks found in the human body. Depending whom you ask, a protein-to gain its moniker-must have more than 20, 40, or 50 amino acids; an average protein in the human body, though, is much larger than this, with somewhere around 500 amino acids. A peptide is any string of at least two amino acids that has fewer than this designated cut-off-a few dozen building blocks rather than a few hundred.
The differences between proteins and peptides, while they are rooted in size, do not end with how large or small they are. Because peptides are so short, they generally do not fold into complex structures like larger proteins; instead of forming helices, sheets, large complexes, and globules, peptides remain a loose, two-dimensional string inside cells. And because of both their small size and lack of organized structure, peptides tend to be able to sneak through spaces where larger proteins can’t fit-they can penetrate the walls of the intestines, human skin, and in some cases even the membranes surrounding cells. For drug makers or food engineers, this is one of the most appealing qualities of biopeptides; they can quickly get to the bloodstream to go where they are needed. In addition, short peptides can be simpler and cheaper to produce compared to complicated, larger proteins.
To make proteins, machinery inside a cell’s nucleus first transcribes a strand of DNA (deoxyribonucleic acid) into a corresponding strand of messenger RNA (ribonucleic acid; mRNA). Next, in association with ribosomes outside of the nucleus, each triplet of m RNA, or codon, is translated into an amino acid that makes up a growing protein. Finally, the protein might be processed by the cell through the addition of chemical entities on the string of amino acids or the removal of sections of the strand.
When it comes to peptides, a few are produced through this classical protein-making pathway. But the vast majority come from elsewhere: the food we eat. When you drink a glass of milk or chow down a burger, your digestive system is faced with thousands of proteins from the food. In the process of digestion, many of these proteins are broken down into smaller, manageable chunks: peptides. Some of these have no immediate function-they will go on to be further processed so the body can later use their building blocks. But others are bioactive peptides; bioactive in the sense that they can cause a change to a cell’s functioning. It is these that have caught researchers’ attention.
Fighting Microbes
Twenty years ago, molecular biologist Maxwell Hincke of the University of Ottawa (Ontario, Canada) started studying the proteins that make up eggshells. He wanted to see whether studying how eggshells form could help inform research on teeth and bones. But he soon realized that the eggshell had even more potential than being a simple model system for calcification. Hincke recently spoke about the proteomic analysis of eggshell membranes at the 106th AOCS Annual Meeting and Industry Showcases (AM&IS) held May 3-6, 2015, in Orlando, Florida, USA.
“As the years went by, I saw that the eggshell isn’t just a passive barrier,” says Hincke. “If you step back, you see that the eggshell has all these mechanisms for protecting the life inside it.”
His group started isolating molecules from the shells and studying their functions and discovered that eggshells-which are discarded by the ton at egg factories-are a plentiful source of bioactive peptides.
In particular, Hincke and his colleagues have homed in on eggshell biopeptides that fight microbes. “This is a completely novel source of antimicrobial peptides, so we’re getting a different kind of insight than ever before,” says Hincke. While all animals have proteins called beta-defensins that help fight disease, for instance, Hincke has unearthed a new beta-defensin that is unique to eggs and may be able to treat bacterial infections that have classically resisted antibiotics.
The finding is an example of why it is so important to isolate and characterize biopeptides from new sources, Hincke says. “Nature is a huge experimental engine for developing molecules with diverse properties,” he says. “We can always go to the lab and manufacture synthetic peptides, but it speeds things up if first we can identify a natural mechanism [that] is the result of millions of years of evolution.”
Often, Hincke says, peptides or proteins can be studied to find the shortest sequence of amino acids-within the larger molecule-that is required for something like an antimicrobial function. “One of our goals is always to define the minimal sequence activity,” he says. “Then we can play around with that sequence to optimize the function.”
Eggshells aren’t the only place where scientists have found antimicrobial biopeptides. Hincke has now isolated some from chicken blood, and others have pinpointed them in the breakdown products of cow and goat milk, beef, and whey. Each works a different way: some bust a microbe’s outer membrane, whereas others interfere with the microbe’s production of DNA or proteins. Some work against nearly all microbes, while others are more specific-a peptide from goat milk cheese, for instance, has been found to improve the symptoms of people infected with Helicobacter pylori.
There are likely more antimicrobial peptides that have yet to be discovered, Hincke points out. “Everything alive has to be able to resist bacterial invasion,” he says. “And it’s a big world out there.”
Peptide Diet
Because many biopeptides are produced when food is broken down in the gut, it is not surprising that many of these mini-proteins have an effect on satiety, appetite, or how the body digests food. In an age of obesity, heart disease, and diabetes epidemics, it is obvious why scientists would be actively researching these effects of biopeptides.
“Dietary proteins have been known to induce satiety with different degrees but the mechanism has not been clear,” says Hiroshi Hara, a professor of food science and nutritional biochemistry at Hokkaido University in Sapporo, Japan. Hara also presented at the recent AM&IS.
Often, research starts because scientists know that a food source is particularly good at making people feel full-or at lowering blood pressure or cholesterol. Then, researchers try to pin down what proteins or peptides are causing this effect.
For Hara, it began with soybeans. Researchers had shown that a soybean protein called β-conglycinin lowers cholesterol and decreases the incidence of atherosclerosis in mice, and suppresses hunger in humans. Hara wanted to know what shorter peptides within the β-conglycinin protein were responsible for the activity. So-as is standard in trying to isolate biopeptides-Hara’s group digested the β-conglycinin protein in the lab and then tested the smaller fragments for their effectiveness at decreasing appetite in rats. The researchers identified a peptide (β 51-63 peptide) that worked.
“Isolation and identification of the active structures in proteins like this are important to understand the mechanism,” Hara says, and to work toward “application of the peptides for prevention of obesity or therapeutics of diabetes.”
Hara’s group is now studying peptides from other types of beans, as well as from sweet potatoes, which have been shown to temper fluctuations in blood sugar. Other researchers have found that fish and seafood are a plentiful source of bioactive peptides that modulate appetite, blood pressure, blood sugar, or cholesterol.
Toshiro Matsui, a professor of bioscience at Kyushu University in Fukuoka, Japan, has isolated tiny peptides-some only two amino acids long-from soybeans, egg whites, and teas-that apparently help prevent high blood pressure and clogged arteries. Matsui spoke about bioactive peptides at the May 2015 AM&IS.
“The advantage of peptides [over proteins] is their preferable intestinal absorption into our body,” says Matsui. The peptides he has found, he believes, affect levels of calcium inside cells, which is key to how they signal. But other peptides exhibit similar effects by blocking a protein called angiotensin-converting-enzyme (ACE), causing blood vessels to relax. Matsui is still characterizing all the effects of the biologically active amino acid pairs that he has identified.
“Dipeptides could improve kidney dysfunction, suggesting that small peptides have still more unknown potential and physiological actions,” he says. To better understand these roles, he has developed a method of labeling peptides to track their movement through the body.
Both Hara and Matsui say that scientists someday may be able to integrate the peptides they find into pills or even food itself to help prevent obesity and diabetes. But, as is the story for many newly discovered biopeptides, more work is needed to characterize the peptides before that can happen.
Biopeptide Beauty
If you read the front of cosmetic containers-everything from anti-aging facial lotion to shampoo-you might think there is one area where biopeptides are already making a big splash in the commercial world. You will see “peptide-infused eye cream,” “peptide lip therapy,” and “peptide cleansing gel” at the beauty counter. But don’t jump to conclusions just yet.
“In reality, most of the marketing you see for cosmetics-including claims about peptides-is exaggeration,” says Perry Romanowski, a cosmetic chemist, vice president of Brains Publishing, and co-founder of the cosmetics blog The Beauty Brains. He spoke about the use and effectiveness of proteins and peptides in cosmetics at the AOCS AM&IS. In fact, he points out, if cosmetics did have bioactive peptides in them, they would no longer be just a cosmetic; they would have to be regulated by the US Food and Drug Administration (FDA) as a drug. That is because a cream or cleanser that interacts with the skins’ metabolism-which a peptide would need to do to be dubbed “bioactive”-falls under the FDA’s jurisdiction.
But Romanowski does admit the claims-in some cases-aren’t just pulled out of nowhere. There are a number of biologically active peptides that research has shown might have anti-aging or wrinkle-removing properties. They are just not likely to be in over-the-counter creams any time soon. “It is really a gray area,” he says.
Peptides advertised in cosmetics-both those that fail to provide their alleged function and those found in drugs that do-generally fall into one of a few categories. Neurotransmitter peptides, such as the botulinum toxin used in BotoxTM, affect the function of nerve cells and can reduce wrinkles by relaxing muscles in the face. Although Botox has been found highly effective, less potent versions of neurotransmitter peptides found in some new over-the-counter creams do not have much effect so far, Romanowski wrote in a recent blog post on peptides.
Other peptides can act as signaling molecules or enzymes, controlling the production or breakdown of larger proteins in the skin-including proteins such as collagen and elastin, which give skin its firmness. And a final set of peptides, carrier peptides, are said to transport small molecules such as copper and magnesium into the skin.
“Scientists have demonstrated through research that some of these do work,” says Romanowski. A peptide called GHK-copper, for example, has been shown in the lab to make skin more firm. But whether the dose of it in your skin cream has that effect is not clear; and if it does, the FDA and other regulatory agencies around the world might be stepping in to regulate it.
Research on anti-aging peptides, Romanowski says, is moving forward particularly quickly. “I do think products are getting a bit better,” he says. “But there’s also a lot of fluff.”
Where Does Collagen Come In
Among the most recognizable proteins-along with insulin and hemoglobin-is collagen. Collagen is the subject of an active area of peptide research because it is a vital component of skin, tendons, ligaments, cartilage, and muscles in humans. Because of its many roles, researchers have been searching for smaller peptides, within the larger collagen proteins.
At Kyoto Prefectural University in Japan, Professor of Applied Biosciences Kenji Sato is among those isolating small peptides from collagen. He set out to see what peptides were circulating in a person’s bloodstream after ingesting collagen-or gelatin, the melted, unstructured form of the protein found in some foods. But he quickly ran into a problem that others have also run up against: Isolating tiny peptides from blood is tricky.
So Sato recently fine-tuned a technique to isolate the peptides he wanted to study. Using a chemical called phenyl isothiocyanate (PITC), Sato can isolate and identify the peptides that would have been missed using conventional analytical methods.
With this new technique, Sato has found almost a dozen di- and tripeptides that are present in the bloodstream after a person eats collagen. Of these, he has shown that a couple, including hydroxyprolyl-glycine and prolyl-hydroxyproline, can stimulate the growth of new skin cells. Aside from cosmetic uses (there are already collagen peptide “beauty drinks” on the market), Sato hopes that the peptides might eventually have medical use.
“I hope the collagen peptide can be used in the hospital as a ‘medical food’ to improve wound healing,” he says.
Moving Forward
Because peptides generally are the byproducts of digestion-and are, therefore, molecules that the human body has been exposed to for years-researchers hope that they will be found to be safer than new, engineered drugs that may have unexpected effects in the body. If the body has no problems digesting soybeans, they argue, then there is no reason a dose of a peptide that comes from soybeans should cause problems. But even if this is the case, biopeptides that are truly biologically active must be tested in rigorous trials to ensure that they do what they say and are safe for consumers and patients.
The good news for those who want to see biopeptides on their food and pharmacy shelves is that research on the molecules is picking up: the number of new peptides entering clinical trials each year jumped by 1,300% between the 1970s and 2000s.
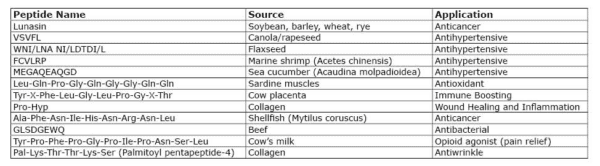
TABLE 1. A sampling of peptides being researched.
Aside from further research on the peptides that have already been identified, work continues on developing new methods to characterize biopeptides by degrading proteins in new ways, separating the resulting fragments in new ways, and screening them for biological function in new ways. Moreover, scientists want to understand better what happens to peptides when they are in the body, using tracking methods such as the labeled peptides Matsui uses. Are there general rules that dictate when a biopeptide can be absorbed by the skin or the intestine, or when a peptide is further degraded instead of circulating in the body? Some guidelines on peptide properties are known, but researchers cannot yet predict the behavior of every peptide.
Every day, we ingest peptides found naturally in the food we eat and produce even more peptides made by our own bodies. So, when a product claims it contains peptides, it is nothing unusual. But, in the future, bioactive peptides may be even more plentiful in the food supply and medicine cabinet. It will just take more research to get there.
Sarah C.P. Williams is a freelance science writer who covers biology, chemistry, and medicine for magazines, newspapers, websites, and institutions around the globe. Her work has appeared in the Los Angeles Times, Science, Nature Medicine, and New Scientist, among many other publications. She can be contacted at sarahcpwilliams@gmail.com.
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…