October 2016
- With increasing legalization of both adult recreational and medical cannabis, there is a need for robust and reliable analytical testing to ensure consumer safety.
- Analytes of interest include cannabinoids, terpenes, residual solvents, pesticides, heavy metals, and microorganisms.
- As lipids, cannabinoids fall within the purview of AOCS. Therefore, AOCS is partnering with industry experts to help develop and validate methods for cannabis analysis and to increase the value of lab proficiency reports.
Cannabis, also known as marijuana, is a flowering plant indigenous to Central and South Asia. The plant has been valued since ancient times for its psychoactive, medicinal, and fibrous properties; however, because of the potential for abuse, coupled with social and political factors, cannabis has been banned in most countries since the early 1900s. In the twenty-first century, this situation appears to be changing. Many countries, including Australia, Canada, North Korea, Columbia, Italy, Spain, and the Netherlands, have decriminalized cannabis possession and cultivation in one form or the other (adult recreational and/or medical use). Although cannabis remains illegal at the US federal level, 25 states plus the District of Colombia allow cannabis use for medical purposes, while four states (Colorado, Washington, Oregon, and Alaska) have legalized cannabis for both medical and adult recreational use.
Complete agreement has not been reached on cannabis’ medical value or the ramifications of legal adult recreational use, but most would concur that cannabis products should be subjected to the same quality and safety tests as any other food or drug on the market. Therefore, testing labs have sprung up to help meet the quality, safety, and labeling requirements for legalized cannabis products in different jurisdictions. But with the legality and acceptance of cannabis use still murky in many locales, such labs have often operated on the fringes of lawfulness, without the benefit of widespread collaboration or guidance from established agencies such as the US Environmental Protection Agency (EPA), Food & Drug Administration (FDA), or Department of Agriculture (USDA) on how to develop and validate analytical methods specific to cannabis products. Despite a rocky start, the cannabis testing industry has matured rapidly in a relatively short period of time, and many competent, certified testing labs are now providing reliable quantitative data to producers and consumers. However, because most cannabis testing labs have developed their own proprietary methods, with little cross-validation among labs, many experts believe that there is a need for standardized analytical methods.
Cannabis components
Cannabis is a genus of flowering plant with compound serrated leaves. The most common species are Cannabis sativa and Cannabis indica. Through selective breeding, growers have developed strains with different sensory, psychoactive, and medicinal properties.

FIG. 1. Glands called trichomes (tiny hairs) on the cannabis flower bud excrete a complex mixture of cannabinoids (including THCa and CBDa), terpenes, and other molecules. Credit: GW Pharmaceuticals
Glands on the cannabis flower buds called trichomes excrete an oily substance containing cannabinoids, terpenes, triglycerides, and other compounds (Fig. 1). More than 480 compounds have been identified that are unique to cannabis, including over 70 cannabinoids (ElSohly, M. A., and Slade, D., https://dx.doi.org/10.1016/j.lfs.2005.09.011, 2005). Cannabis is smoked, cooked, or otherwise heated to produce the two most prevalent cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD), Fig. 2. In the plant, THC and CBD exist in their acid forms, THCa and CBDa. Heat decarboxylates the acid forms to produce THC and CBD. Other cannabinoids such as cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), tetrahydrocannabivarion (THCV), and cannabidivarin (CBDv) are also being isolated and studied.
FIG. 2. Structures of the cannabinoids THC and CBD. Credit: GW Pharmaceuticals
THC is the main psychoactive component of cannabis, whereas THCa (the native form in the plant) lacks psychoactive effects. CBD, which is non-psychoactive, is valued primarily for its medical effects, but CBD may also influence the psychoactive properties of THC. Cannabinoids produce their physiological effects by acting in distinct ways upon cannabinoid receptors, primarily in the brain and immune system. At this time, most evidence of cannabis’ medical efficacy is anecdotal because limited clinical trials have been conducted, but proponents of medical cannabis claim that it can reduce nausea, seizures, inflammation, and pain, and can help treat ailments such as multiple sclerosis, epilepsy, glaucoma, Crohn’s disease, and cancer.
Growers of adult recreational cannabis often try to maximize THC content, as higher levels of THC demand higher prices. Today’s THC levels, often 20% or more relative to the bulk plant material (w/w), are much higher than those in cannabis strains from the 1970s, which contained only 4-6% THC (Ruppel, T. D., Kuffel, N., https://tinyurl.com/PE-cannabis, 2015). Levels of CBD are generally low in recreational strains (e.g., 2% w/w). In contrast, many medical cannabis strains contain higher levels of CBD (e.g., 14%) and lower levels of THC (e.g., 1%), and many strains target specific ratios of the compounds. For medical cannabis patients, the THC “high” may be unnecessary or undesirable, especially when treating children or chronic conditions that require medicating throughout the day.
Cannabis also contains approximately 140 terpenes (Ruppel, T. D., Kuffel, N., https://tinyurl.com/PE-cannabis, 2015). Terpenes, the basis of “essential oils,” are molecules composed of multiple isoprene units and typically have pleasant fragrances. Examples include α–pinene (pine needles, rosemary), myrcene (clove-like, earthy, fruity), limonene (citrus), and linalool (floral). The particular terpene profile of a cannabis strain influences its flavor and fragrance. Different cannabis strains are named for their aromas, e.g., Super Lemon Haze, Grape Skunk, and Girl Scout Cookies. In addition to determining the sensory properties of cannabis, terpenes may enhance medical benefits through a process known as the “entourage effect.”
States’ rights
Because the FDA still classifies cannabis as a Schedule 1 drug (having a high potential for abuse and no accepted medical use; this class also includes heroin, LSD, and ecstasy), the US federal government has mostly taken a “hands-off” approach to cannabis regulation, leaving these matters to the individual states that have legalized the substance. The exception is cannabis products that make medical claims, which are forbidden without prior FDA approval. Cannabis-based drugs that claim therapeutic effects must go through the same lengthy FDA approval process as other drugs, including clinical trials for safety and efficacy.
Although the FDA has not approved cannabis for any medical use, the agency has approved two drugs (Marinol and Syndros) that contain a synthetic form of THC (https://tinyurl.com/FDA-medical-marijuana). Both drugs were approved for the treatment of anorexia in AIDS patients and for nausea and vomiting associated with cancer chemotherapy in patients who did not respond to conventional treatments.
Cannabis testing requirements vary by state, but most states require testing and labeling for potency (THC and CBD) and various contaminants such as residual solvents, microbes, heavy metals, and pesticides. For example, in Colorado, all retail cannabis products must be tested for potency (THC, THCa, CBD, CBDa, and CBN), residual solvents (butane, hexane, heptane, and BTX), and microorganisms (E. coli, Salmonella, yeast, and mold) before hitting dispensary shelves. As yet, testing for heavy metals and pesticides is not mandatory, but the Colorado Marijuana Enforcement Division has set limits for these contaminants that could be verified by random testing.
Potency tests
The primary cannabinoids of interest for potency tests are THC, CBD, and CBN. A breakdown product of THC, CBN is an indicator of cannabis deterioration due to age or poor storage conditions. The two most common methods for potency analysis are high-performance liquid chromatography (HPLC) with UV detection and gas chromatography (GC) with flame ionization detection (FID) (Table 1). Although GC is more cost-effective and simpler than HPLC, this method requires sample derivatization to quantitate both the free and acid forms of THC and CBD. This is because the heat necessary for GC sample injection converts THCa into THC, and CBDa into CBD. Therefore, without deriviaization, the free and acid forms cannot be distinguished or quantified.
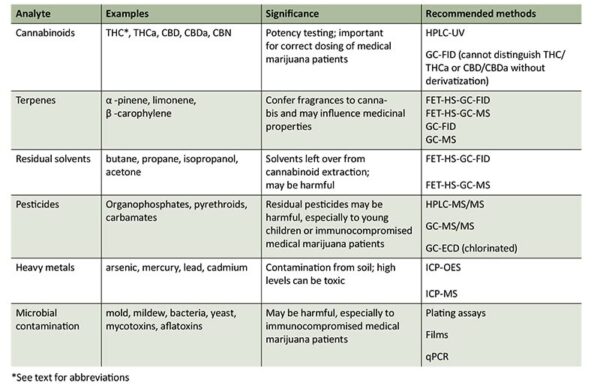
TABLE 1. Recommended methods for cannabis analysis.
Derivatization methods are highly subject to error and difficult to validate, so many labs are choosing to invest in LC equipment. In a recent lab proficiency testing program, a survey of preferred potency testing methods found that 90% of the labs use LC (Emerald Test lab proficiency program, Emerald Scientific, San Luis Obispo, Calif., USA). However, GC without derivatization can provide a “quick and dirty” estimate of cannabinoid potency (THC + THCa, CBD + CBDa), which may be helpful for process monitoring.
In contrast, HPLC can separately quantify THC, THCa, CBD, and CBDa without derivatization, which is particularly useful for edible cannabis products because they will typically be consumed without additional heating. For edibles, GC might provide erroneously high potency values because the technique itself converts THCa into THC, and CBDa into CBD. Several companies, including Ceriliant (Round Rock, Tex., USA), Emerald Scientific, and Restek (Bellefonte, Penn., USA) offer cannabinoid standards to help identify and quantify peaks in GC and HPLC.
The desire to identify additional cannabinoids has led to wider use of two more sophisticated chromatographic techniques, Ultra Performance Chromatography (UPC) and Supercritical Fluid Chromatography (SFC). Compared to HPLC, UPC has the advantage of a higher separation efficiency, which results in better resolution, shorter analysis times, and reduced consumption of mobile phase (and therefore, less generation of hazardous waste). SFC has all of the advantages of UPC, combined with much easier sample preparation. SFC is very amenable to non-polar diluents, in which lipophilic cannabinoids are highly soluble. This attribute is particularly useful for isolating cannabinoids from the large variety of matrices available for cannabis-infused products.
Portable devices are also being used for potency tests. Fourier Transform infrared spectroscopy (FTIR) can provide quick and easy potency spot tests for THC, THCa, CBD, and CBDa in dried cannabis buds and processed oils. Although not as sensitive as chromatography, FTIR can analyze whole buds for potency, terpenes, and moisture content. Because the technique is not a primary method, standard samples are needed with known concentrations determined by other techniques, such as GC or HPLC.
Terpenes
Terpenes present an analytical challenge because they are nonpolar and structurally similar, and many structural isomers exist. Mass spectrometry (MS) cannot distinguish terpenes that co-elute from a GC column because many have the same molecular weight and share fragment ions. While working at Restek, Amanda Rigdon (now chief technical officer at Emerald Scientific, San Luis Obispo, Calif., USA) and colleagues developed a method to analyze terpenes in cannabis using full evaporation technique (FET)-headspace (HS)-GC-FID (Rigdon, A, et al., https://tinyurl.com/Restek-terpenes, 2014).
In FET-HS-GC-FID, a small amount of cannabis sample (20 mg or less) is placed in a 20-milliliter headspace vial and heated to volatilize terpenes in the sample into the gas phase, or headspace. The FET-HS technique is particularly useful for analyzing volatile components due to its ease of implementation and minimal sample processing. Once in the HS, the terpenes are injected onto the GC column where they are separated and then detected by FID. Less-volatile cannabinoids mostly remain in the sample, preventing them from overwhelming the less-abundant terpenes on the GC column. Using FET-HS-GC-FID, Rigdon and colleagues were able to profile 38 terpenes found in cannabis. However, the method is only semi-quantitative due to the relatively low volatility of some of the terpenes, as well as adsorption effects in solid matrices.
Residual solvents
The extraction of cannabis to produce materials for use in oils, edibles, and other products often utilizes solvents such as butane, propane, isopropanol, or acetone. These solvents are harmful to health, so they should be absent from the final product. There is a trend in the industry to move away from these toxic solvents and employ supercritical carbon dioxide, ethanol, or water in extraction procedures. Because solvents are volatile, FET-HS-GC-FID can be used for both terpene and residual solvent analysis, says Rigdon. However, peak identification requires that the cannabis producer accurately reports which solvents were used in the extraction.
GC/MS can accurately identify peaks without prior knowledge of solvents; however, MS has a linear dynamic range that makes it difficult to analyze solvents that vary widely in concentration. “If you look at a lot of the state regulatory lists, the action level for butane is anywhere from 800 ppm to 5,000 ppm, whereas benzene is 1 to 2 ppm,” says Rigdon. “So if you were operating at a sensitivity high enough to see 1 ppm of benzene, you’re going to be overloading your detector with butane, because mass spectrometers overload much more quickly than FIDs.”
Pesticides
It is illegal for cannabis growers to use pesticides and fungicides to control aphids, spider mites, and mold, which thrive in the warm, moist indoor conditions used to grow cannabis, unless such use is listed on the manufacturer’s label. Currently, there are no insecticides that list cannabis on the label, which puts some growers in a desperate situation. In trying to save their crop, they may choose to break the law and use compounds that are forbidden. State regulations on pesticides vary, but some agencies have defined pesticide-positive samples as those containing 0.1 ppm of any pesticide. There are thousands of known pesticides, so it is currently impossible to test for all. Oregon regulators have selected a 59-pesticide panel for the state’s testing requirements. “Even with 59 pesticides, it’s impossible for one chromatographic system to analyze them all effectively,” says Rigdon.
GC with an electron capture detector (ECD) can detect chlorinated pesticides at the parts-per-trillion (ppt) level, but the technique cannot detect non-chlorinated pesticides. GC in combination with tandem mass spectrometry (GC-MS/MS) can detect many pesticide classes. Although much more complex than GC-ECD, GC-MS/MS has an advantage for “dirty” samples such as edibles due to the selectivity of the triple quadrupole detector. HPLC-MS/MS can be used for many pesticide classes, as well, and is required for the analysis of heat-labile pesticides such as Abamectin.
“If I was going to buy one instrument for pesticides, it would be an HPLC-Triple Quad [MS/MS],” says Rigdon. “Ninety-five percent of the pesticides out there can be analyzed by HPLC-MS/MS, although there are some that you would need a GC-MS/MS for.”
For edibles, sample cleanup is essential prior to pesticide analysis by either type of MS/MS. A popular sample preparation method originally developed for analyzing pesticides in fruits and vegetables is QuEChERS (quick, ease, cheap, effective, rugged, and safe). QuEChERS can remove particulates, fats, and sugars in cannabis edibles that can foul chromatography columns or otherwise interfere with analyses. In QuEChERS, the edible sample is hydrated and homogenized with a tissue lyser or cryogenic grinder to produce very fine particles. Then, the sample is extracted with acetonitrile, and an extraction salt packet is added to cause partitioning. The resulting acetonitrile layer is then cleaned up using either dispersive solid phase extraction (dSPE) or cartridge SPE (cSPE). The cleaned-up sample can then be loaded onto an HPLC-MS/MS or GC-MS/MS instrument.
A couple of recent studies have exposed high levels of pesticides in cannabis products. As reported in a Spokane, Wash., USA, newspaper, The Spokesman-Review, Trace Analytics, a cannabis testing lab in Spokane, tested dozens of cannabis flowers and concentrates purchased from Washington dispensaries (https://tinyurl.com/TA-pesticides). The lab found many products with pesticides in the ppm range, well above proposed limits. (Like most states with legalized cannabis, Washington currently lacks official pesticide testing requirements.) OG Analytical, a cannabis testing lab in Eugene, Ore., USA, recently discovered that a “pesticide-free” plant wash used heavily in the cannabis industry actually contains an illegal pesticide not registered with the U.S. Environmental Protection Agency (EPA). “It turned into a huge lawsuit,” says Rodger Voelker, lab director at OG Analytical. “That was sort of a wakeup call, and people started sending us lots of different products to test to make sure they don’t actually have pesticides in them.”
Voelker says that OG Analytical specializes in pesticide testing, but not every testing lab is set up to do the complex analyses. “Pesticides are by far the hardest analyses that are going to be done in the cannabis industry,” says Rigdon. “When it comes to screening, we’re getting pretty close, but actual quantitative testing is going to take a while. The food safety industry has had decades to develop their methodologies, and they’re still wrestling with pesticide testing in complex matrices.” She adds that a validated method for analyzing pesticides in a brownie is not going to work for pesticides in a gummy bear, soda, pasta or any of the other hundreds of matrices available for cannabis.
Heavy metals
Heavy metals such as arsenic, mercury lead, cadmium, and chromium can enter cannabis plants from contaminated soil. These metals can be detected at trace amounts (ppt) by inductively coupled plasma (ICP)-MS or ICP-optical emission spectrometry (OES). Like techniques for pesticide analysis, methods for heavy metal analysis parallel those used by the food industry.
Microorganisms
During growth or storage, cannabis plants can become contaminated with microorganisms such as mold, mildew, bacteria, and yeast. Pathogenic bacteria such as Escherichia coli and Salmonella, as well as fungal toxins such as mycotoxins and aflatoxins, can cause severe illness, particularly in children or immunocompromised patients who are taking medical cannabis.
“Our micro testing is actually two different kinds of tests,” says Lucas Mason, co-founder and lead analyst at Aurum Labs, a cannabis testing lab in Durango, Colo., USA. “We test for pathogenic bacteria using qPCR [quantitative polymerase chain reaction], which is quick. Then we plate samples on standard media and get a total yeast and mold count after about three days.” Petri film techniques can also be used for microbiological analyses. “We started very old school, just standard plates, and that was really valuable for us,” says Mason. “We learned a lot about what grows on cannabis because there’s really not a lot out there in academia. We now have a library of about 30 common species that are growing across all of our clients, all of our regions.
Cannabis fingerprinting
As could be expected, pharmaceutical companies that market cannabis-based drugs typically conduct much more rigorous testing than producers of adult recreational cannabis products. GW Pharmaceuticals, a company based in Cambridge, UK, is developing a portfolio of cannabinoid-based medicines. One of these, Sativex, has been approved in the UK and 24 other countries (although not yet in the United States) for the treatment of multiple sclerosis-related muscle spasms. To make Sativex, GW Pharmaceuticals combines extracts from two different cannabis strains, one high in THC and the other high in CBD, to yield a final THC:CBD ratio of 1. The company grows each cannabis strain under tightly controlled conditions (including no pesticides) in separate facilities (Fig. 3). Then, they make extracts from each strain and combine the extracts.

FIG. 3. At GW Pharmaceuticals, cannabis plants are grown indoors under tightly controlled conditions to minimize chemical variability. Credit: GW Pharmaceuticals
GW Pharmaceuticals wanted to perform quality control tests at multiple steps in the process, so they contracted a company called Infometrix to help with the analysis. Infometrix, based in Bothell, Wash., USA, develops chemometrics software tools and services for various industries. “Chemometrics is kind of like fingerprint analysis, only it’s a fingerprint of a chemical system,” says Brian Rohrback, president of Infometrix. “We’ve built custom quality control systems for a variety of applications, but they all have one thing in common: You’ve got complex data with a lot of correlations, and you have to remove those correlations to find out what’s happening chemically.”
GW Pharmaceutical’s goal was to ensure batch-to-batch consistency for Sativex, a difficult feat for botanical extracts. So in collaboration with Infometrix, they divided the extracts from the two cannabis strains into four fractions each (cannabinoids, terpenes, sterols, and triglycerides), and analyzed the constituents in each fraction three times by HPLC or GC (24 analyses). Then, they combined the extracts and analyzed the cannabinoids and terpenes again three times (six analyses). “So the issue for us was, how do you combine these thirty analyses into red light/green light, pass/fail, do we sell this or not?” says Rohrback.
To develop their chemometrics system, Rohrback and his colleagues examined six years’ worth of GW Pharmaceutical’s quality control data. “We built ten PCA [principal components analysis] models, one for each of the four fractions in the two cannabis strains and for the two fractions in the mixture,” says Rohrback. “When we analyze a new batch, we can compare it against the models and say whether, statistically significantly, the batch falls within the 95% confidence interval” (Fig. 4).
Growing pains
As more US states legalize cannabis, the industry continues to grow, and continues to feel bumps along the way. Many growers of cannabis had no previous experience in farming, so they made mistakes like using pesticides illegally or growing bumper crops of fungi. On the analytical side, people with liberal arts degrees or no college education joined the ranks of Ph.D. analytical chemists, sometimes with less-than-satisfactory results. “What I’ve observed is a lot of people who’ve gotten their education on what to do for quality control from the internet,” says Rohrback. “They will go off and buy an analytical instrument on eBay and try to set it up, when they know nothing about the instrumentation or how to repair it.”
Another challenge is representative sampling. Because cannabis is a valuable commodity, producers are often reluctant to provide more than a gram or two of sample for testing. Sometimes they will send only a single cannabis bud, which is hardly representative of, say, a 250-pound crop. “When there’s no third-party sampling, some people will cherry pick,” says Mason. “They’ll find the needle in the haystack and try to send me their best-looking bud. As a result, their potency numbers will bounce around, and they think it’s my fault.” Because products with a higher THC content can demand a higher price, lab shopping is a problem, says Mason.
Sample tampering is also an issue, says Cynthia Ludwig, director of technical services at AOCS. Some growers will roll buds selected for potency testing in a concentrated form of cannabis extract known as kief, which is 30-50% cannabinoid by weight, to boost their THC values. Or they will try to influence the microbiological tests. “In my discussions with labs doing microbial testing, I’ve heard stories of people who figured out how to cheat the microbial test by putting their samples in the microwave,” says Ludwig. “Clients will send two samples to the lab, supposedly from the same batch-except the potency sample is green and beautiful, and the micro sample is brown, dry and crispy.”
Standardized methods needed
Currently, there are no standardized methods for cannabis analysis. As a result, each lab selects or develops its own methods to meet state testing requirements. According to Voelker, there is little collaboration among labs. “It’s a complete crap shoot,” he says. “Nobody shares anything, and everybody thinks they’re the only ones doing a good job.”
“A proprietary method is not a competitive advantage. It’s a danger to medical cannabis patients,” says Ludwig. “If everybody has their own proprietary method, then you don’t know which one’s correct, and it’s hard to determine the correct THC dose for patients.” To develop validated methods for cannabis analysis, AOCS has assembled a Cannabis Expert Panel of 75 top analytical scientists and cannabis industry professionals. The panel is helping to identify analytes of interest and the most accurate technologies for determining their levels.
“We currently have five methods that are in the midst of validation, and the hope is that they will be widely used by cannabis analytical labs,” says Ludwig. These include two methods for cannabinoid analysis, a method for residual solvent analysis, one for heavy metals, and a sample preparation method. Ludwig expects validation data by the end of 2016, which will be followed by 3-6 months of collaborative studies. “We’re hoping for the first methods to be available for use in Summer 2017,” she says.
In addition, AOCS has partnered with Emerald Scientific, a supplier of cannabis testing products, to provide ISO 13528-compliant reports for the Emerald Test lab proficiency program. Interested labs receive samples that they test for potency and residual solvents, and they enter their data in an electronic portal. Then, AOCS analyzes the data using its established lab proficiency program. “The participants receive a report showing all of the participants’ anonymous results, and they can see how they stack up against other analytical labs testing the same sample,” says Ludwig. In addition to the raw data, the report includes the consensus mean and z-scores, as well as kernel density plots, so that participants can visualize where their lab falls within the group.
The maturing of an industry
In the past few years, the cannabis industry has matured from naïve exuberance to a more staid and reliable approach that craves legitimacy. “In the very beginning, there were a couple of labs where you paid one amount for the true potency value, and you paid another amount for something over 20%,” says Mason. “So the precedent that the labs are shady and number factories was set pretty early on, and I think that sowed the seeds of distrust.”
But this situation is changing, says Rigdon. “This industry is unique because everybody is so passionate and driven. They’re trying their best,” she says. “It’s good to see them getting some wider recognition. I think that will bring them into the scientific community as a whole, which is where they need to be. They’re not on the fringes anymore. They’re a true analytical industry.”
Laura Cassiday is an associate editor of INFORM at AOCS. She can be contacted at laura.cassiday@aocs.org.
Information
- ElSohly, M. A., and Slade, D. (2005) “Chemical constituents of marijuana: the complex mixture of natural cannabinoids.” Life Sci. 78, 539-548. https://dx.doi.org/10.1016/j.lfs.2005.09.011
- Hill, K. “Spokane lab finds pesticides in many marijuana products.” The Spokesman-Review, Sunday, March 20, 2016. https://tinyurl.com/TA-pesticides
- Rigdon, A, et al. (2014) “A preliminary FET Headspace GC-FID method for comprehensive terpene profiling in cannabis.” https://tinyurl.com/Restek-terpenes
- Ruppel, T. D., and Kuffel, N. (2015) “Cannabis analysis: potency testing identification and quantification of THC and CBD by GC/FID and GC/MS.” PerkinElmer Application Note. https://tinyurl.com/PE-cannabis
- U.S. Food & Drug Administration. (Page Last Updated 08/01/2016) “FDA and Marijuana: Questions and Answers.” https://tinyurl.com/FDA-medical-marijuana
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…
Lipid Library
Hempseed oil in a nutshell
By J.C. Callaway March 2010 Industrial hemp is as a class of…