By Laura Cassiday
April 2014
After reading this article, you will understand:
- the basic science of emulsions;
- how formulators choose which emulsifier to use for a particular emulsion;
- how emulsifiers are used in foods, nutraceuticals, personal and home care products, industrial lubricants, environmental technologies, biofuels, and other applications.
The immiscibility of oil and water has inspired the proverb “Oil and water don’t mix” and other expressions that reflect the general incompatibility of two entities, such as “My coworker and I are like oil and water.” Yet within our homes are numerous examples of products in which oil and water do mix: mayonnaise, milk, salad dressings, hand lotion, and hair conditioner, to name but a few. These examples represent emulsions, which are stable mixtures of tiny droplets of one immiscible fluid within another, made possible by chemicals called emulsifiers.
How emulsions and emulsifiers work
Simple emulsions are either oil suspended in an aqueous phase (o/w), or water suspended in oil (w/o). Milk is an example of an o/w emulsion, in which the fat phase or cream forms tiny droplets within the skim milk, or water phase. In contrast, margarine is a w/o emulsion containing droplets of water or skim milk in a blend of vegetable oils and fat. In both cases, emulsifiers are needed to prevent the suspended droplets from coalescing and breaking the emulsion.
Anybody who has made a simple oil-and-vinegar salad dressing knows that, with enough shaking or whisking, one can make a temporary emulsion. However, in the absence of emulsifiers, this unstable emulsion breaks down within minutes, and the oil forms a layer on top of the vinegar. For centuries, cooks have added natural emulsifiers, such as egg yolk, mustard, or honey, to help prevent this separation. Today, a wide variety of nature-based and synthetic emulsifiers are available for the diverse fields that benefit from them, including food, nutraceuticals, home and personal care, biofuel, environmental cleanup, and industrial lubricant applications.
Emulsifiers work by forming physical barriers that keep droplets from coalescing. A type of surfactant (see Sidebar), emulsifiers contain both a hydrophilic (water-loving, or polar) head group and a hydrophobic (oil-loving, or nonpolar) tail. Therefore, emulsifiers are attracted to both polar and nonpolar compounds. When added to an o/w emulsion, emulsifiers surround the oil droplet with their nonpolar tails extending into the oil, and their polar head groups facing the water (Fig. 1). For a w/o emulsion, the emulsifier’s orientation is reversed: nonpolar tails extend outward into the oil phase, while polar head groups point into the water droplet. In this way, emulsifiers lower the interfacial tension between the oil and water phases, stabilizing the droplets and preventing them from coalescing.
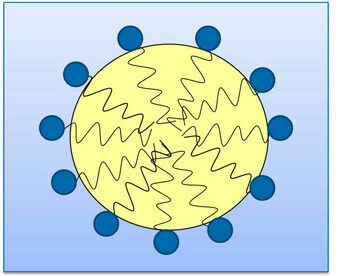
FIG. 1. When a surface-active emulsifier is used to combine water and oil, the polar head group (shown in the blue circle) is attracted to water, while the non-polar tail (wavy black line) is attracted to oil, allowing the water and oil to combine. Courtesy of The Lubrizol Corp. (Wickliffe, Ohio, USA); reprinted with permission from Tribology & Lubrication Technology 69(9):32-30 (2013).
Emulsifiers can be cationic (positively charged polar head group), anionic (negatively charged head group), or non-ionic (uncharged head group). When charged emulsifiers coat droplets in an o/w emulsion, the positive or negative charges on the outside of the oil droplets electrostatically repel each other, helping to keep the droplets separated. Non-ionic emulsifiers tend to have large, bulky head groups that point away from the oil droplet. These polar head groups clash and tangle with head groups on other water droplets, sterically hindering the droplets from coming together. The type of emulsifier used depends on the application, with cationic emulsifiers typically used in low-to-neutral pH solutions and anionic emulsifiers in alkaline solutions. Non-ionic emulsifiers can be used alone or in combination with charged emulsifiers to increase emulsion stability.
How to choose the right emulsifier
How do product formulators choose which emulsifier to use for a particular emulsion? Calculating the hydrophilic-lipophilic balance (HLB) of an emulsifier or combination of emulsifiers can help. In an ideal emulsion, the emulsifier is equally attracted to the water phase and the oil phase. If the balance is tipped in either direction, the emulsifier may lose contact with the phase to which it is less attracted, causing the emulsion to break down.
Different emulsifiers have different HLB values, which can predict their ability to stabilize various kinds of emulsions (Fig. 2). The HLB scale ranges from 0 to 20, with 10 corresponding to an emulsifier that is equally attracted to water and oil. Emulsifiers with HLB values greater than 10 are more hydrophilic and thus better at stabilizing o/w emulsions. In contrast, emulsifiers with HLB values less than 10 are more hydrophobic and therefore better suited for w/o emulsions.
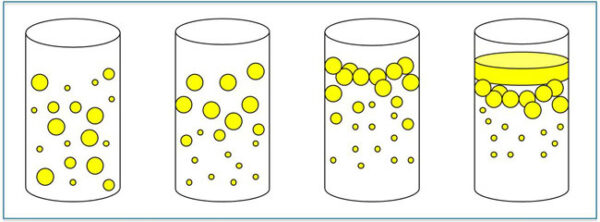
FIG. 2. Emulsions offer different stability, and applications demand varying levels of emulsification stability. From left to right: a stable emulsion; the emulsion had begun to separate; the emulsion is creaming (thick white layer on top of the mixture); the emulsion has broken (noticeable oil layer on top of the water phase). Courtesy of The Lubrizol Corp. (Wickliffe, Ohio, USA); reprinted with permission from Tribology & Lubrication Techology 69(9):32-29 (2013).
Furthermore, different oils have different HLB requirements. For example, vegetable oil emulsions need an emulsifier with an HLB of 7–8, whereas the required HLB value to form a stable castor oil emulsion is 14. By matching the HLB value of the emulsifier with that of the oil, formulators can greatly increase their chances of producing a stable emulsion.
According to George Smith, technical director for the Americas at Huntsman Performance Products in The Woodlands, Texas, USA, a combination of emulsifiers usually works better than any single emulsifier. “If you’re trying to make a mineral oil emulsion, for example, the HLB for mineral oil is 10,” he says. “So you’ll pick a pair of emulsifiers, one with an HLB higher than 10 and another with an HLB lower than 10. When you combine them, the average comes out around 10.”
The HLB system, which works primarily for non-ionic emulsifiers, has been around since 1954. In the 1970s, the hydrophilic-lipophilic difference (HLD) system was introduced. The HLD system works for ionic as well as non-ionic surfactants, and it is better able to take into account detailed characteristics of a particular emulsion such as salinity, oil type, surfactant concentration, and temperature.
The HLD equation includes terms for the salt concentration, “oiliness” of the oil (the effective alkane carbon number), and the characteristic curvature (Cc) of the emulsifier. The Cc value of an emulsifier reflects whether the emulsifier prefers to curve around an oil droplet in water (negative Cc) or to curve around a water droplet in a w/o emulsion (positive Cc). For example, a very hydrophilic emulsifier, sodium laurel sulfate, has a Cc of –2.3, whereas a very hydrophobic emulsifier, dioctyl sodium sulfosuccinate, has a Cc of 2.6. The Cc for combinations of emulsifiers is the weighted average for each emulsifier. The HLD scale centers on 0, which corresponds to the optimal emulsion. Online calculators exist to optimize the HLD for a particular emulsion (e.g., www.stevenabbott.co.uk/HLD-NAC.html).
Macro- and microemulsions
Increasingly, formulators are interested in making microemulsions, which offer greater stability than conventional macroemulsions. As the name suggests, microemulsions have smaller droplet sizes than regular emulsions, making them appear transparent rather than opaque. Unlike macroemulsions, microemulsions are thermodynamically stable. “Given enough time, a macroemulsion will break down into water and oil phases,” says David Sabatini, associate director of the Institute for Applied Surfactant Research at the University of Oklahoma, Norman, USA. “But time is not a factor in how long a microemulsion will remain in its current state.” In addition, if a temperature change causes an emulsion to break down, a microemulsion will spontaneously reform when the temperature changes back to its original value. In contrast, a macroemulsion requires an energy input to reappear.
Microemulsions are made differently from macroemulsions. Macroemulsions require high-intensity mixing. Because microemulsions are a thermodynamically stable end point that a system naturally migrates toward, they generally do not require vigorous mixing. However, formulators often use gentle agitation to evenly spread the components and speed up the process of microemulsion formation.
Compared to macroemulsions, microemulsions require more surfactant. “Time stability points in the direction of microemulsions, but surfactant requirement may point in favor of macroemulsions,” says Sabatini. “It may be that 3 or 6 months is plenty long enough for your application and time may not be a factor in that situation.” For example, food products will often go bad before a macroemulsion breaks down, he says.
Because of their remarkable stability, microemulsions are finding applications in diverse fields such as personal care products, oil field chemicals, and medicine. “Macroemulsion concepts have been around for centuries, but advanced microemulsion concepts are only about two to three decades old,” says Sabatini. “There’s growing interest in microemulsions because we’re just beginning to understand their capabilities.”
Foods
Many popular food items are emulsions, including mayonnaise, salad dressings, sauces such as Hollandaise, chocolate, and ice cream. Lecithin, a blend of naturally occurring phospholipids, is widely used in the food industry to promote o/w emulsions. Worldwide, most commercial lecithin comes from soybean oil. Egg yolk, the traditional emulsifier for mayonnaise and sauces, also contains lecithin. Other common emulsifiers in foods are proteins, fatty acid esters, sodium stearoyl lactylate, and mono- and diglycerides.
Making food emulsions can be challenging because “foods are complex systems with many different ingredients interacting,” says John Neddersen, senior application scientist in fats, oils, and emulsifiers at DuPont Nutrition and Health, based in New Century, Kansas, USA. “Although guidelines like the HLB scale can help, most of the time experience and experimentation are needed to find the optimal choice of emulsifiers and usage rates.” Neddersen notes that processing can be another challenge when working with food emulsions. “A company might have a single formula run at multiple locations and see different results at the different plants,” he says. These differences may arise from seemingly subtle variations in plant conditions.
DuPont sells a broad range of emulsifiers, including the Panodan® DATEM (diacetyl tartaric acid ester of monoglycerides) line especially for bakery products and the Cremodan® line for ice creams and other frozen desserts. As an alternative to lecithin in chocolates and other confectionary, DuPont offers Grindsted® CITREM, a citric acid ester. This emulsifier can substitute for soy lecithin, which has recently come under fire, particularly in Europe, because most soy crops grown for export (especially the United States, Brazil, and Argentina) are genetically modified. Non-genetically modified soy is expensive and in short supply. Therefore, CITREM may prove an attractive alternative for confectioners who want to avoid ingredients made from genetically modified soy.
Sustainable sourcing of palm oil has also become a customer concern, as reports have surfaced that the development of palm oil plantations harms the environment and threatens endangered wildlife in Malaysia and Indonesia, where most palm oil originates. As a result, DuPont introduced a portfolio of emulsifiers based on sustainably sourced palm and non-palm oils. By 2015, DuPont has pledged to source 100% of its palm oil from plantations certified by the Roundtable on Sustainable Palm Oil (RSPO).
Reduced-fat emulsions are another hot topic for the food industry. When fat is removed from a food to make a reduced-fat or fat-free version, the taste, appearance, and texture often suffer. D. Julian McClements, professor of physico-chemistry at the University of Massachusetts Amherst, USA, says that there are several ways that emulsions or emulsifiers could help reduce the fat content of foods. For instance, researchers could structure water-in-oil-in-water (w/o/w) emulsions. “You could take some of the fat out of the droplets and replace it with water,” he says.
Another approach, called heteroaggregation, is to mix oil droplets coated with emulsifiers of opposite charge. “We mix a positive droplet and a negative droplet together, and they form a gel network,” says McClements. “The resulting emulsion has a very high viscosity and low fat content and mimics some of the characteristics of a high-fat product.”
Nutraceuticals
Researchers are exploring emulsions as delivery vehicles for vitamins, supplements, and other nutraceuticals. McClements’ lab has used emulsions to encapsulate vitamin E, carotenoids, omega-3 fatty acids, curcumin, coenzyme Q10, and other bioactive compounds. Eventually, he would like to incorporate nutraceuticals such as these into functional foods.
“One of our goals is to increase the stability of active compounds that are encapsulated in emulsions in food particles,” says McClements. “We’d also like to control their fate in the gastrointestinal tract once they’ve been digested.”
In addition to conventional emulsions, McClements’ lab makes more complex emulsions such as nanoemulsions, solid-lipid nanoparticles, filled hydrogel particles (Fig. 3), and multilayer emulsions. Different types of emulsions could have different applications. “Some of them can protect components from chemical degradation, some can deliver compounds to the colon, and some can control flavor release,” says McClements. “So you have to have a different kind of delivery system for each application.”

FIG. 3. Filled hydrogel particles in foods can deliver nutraceuticals to the body. The filled hydrogel microspheres consist of tiny fat droplets trapped within larger spheres made from food biopolymers. The fat droplets may contain nutraceuticals, such as vitamins, carotenoids, or curcumin. This is an example of an o/w/w emulsion, since the hydrogel particles are mainly water, and they are surrounded by water. Courtesy of D. Julian McClements, University of Massachusetts Amherst.
Multilayer emulsions consist of oil droplets coated with an emulsifier plus one or more biopolymer layers, dispersed in an aqueous solution. The emulsifier is typically electrically charged, and the polymer layer(s) have opposite charges that attract them to the surface of the oil droplet.
According to McClements, multilayer emulsions tend to have better physical stability than single-layer emulsions through fluctuations in pH, ionic strength, temperature, freezing and thawing, and dehydration. In addition, researchers can design multilayer emulsions to control their breakdown in the gastrointestinal tract. “You can make them so they’re digested very quickly, like a normal emulsion, or you can make them so they go further down the gastrointestinal tract,” he says. “The latter might be useful if you want to deliver something to the colon or you’re trying to control satiety by getting undigested compounds further down in the gastrointestinal tract.”
Personal care
Most personal care products, including lotions, creams, shampoos, and conditioners, are emulsions. Common emulsifiers for personal care products include ethoxylated alcohols, carboxylates, sodium isethionate, glycerol monostearate, cetyl alcohol, stearyl alcohol, and silicone emulsifiers such as dimethicones.
“The trend right now is most people would like to use an emulsifier that’s based on plant raw materials rather than petrochemicals,” says Smith. Synthetic emulsifiers such as ethoxylated alcohols and their naturally derived counterparts have identical structures, performance, and biodegradation. “The price swings back and forth depending on the price of palm kernel oil in Malaysia and the price of ethylene in North America,” says Smith. “At the moment, I think petrochemicals have the advantage, but it switches every two to three years.”
Juan Mateu, technical director at JEEN International in Fairfield, New Jersey, USA, says that there has been a move away from synthetic ethoxylated alcohols in recent years due to worries about residual 1,4-dioxane, a suspected carcinogen that is a by-product in their manufacture. Naturally derived glucosides have been suggested as replacements for some applications. However, “It’s too early to say that ethoxylated alcohols can be replaced,” says Mateu. “There are some emulsions you can make with glucosides, but for the most part the whole world is still using ethoxylates.”
In 2009, JEEN International launched its Jeesperse line of cold-process emulsifiers, which allows formulators to make emulsions containing waxy substances at ambient temperatures (25–30°C). Many common emulsifiers in personal care products, such as cetyl alcohol and glycerol monostearate, are waxes with relatively high melting points (up to 165°C). Prior to Jeesperse, manufacturers had to heat emulsifiers in the oil phase to melt them, and then add the melted emulsifier to the aqueous phase and cool the emulsion at a controlled rate down to room temperature. In contrast, Jeesperse allows the emulsion to be made in a single kettle at room temperature, resulting in significant savings of money and time.
The secret ingredients in Jeesperse products are polyelectrolytes, such as sodium polyacrylate. The polyelectrolytes are polar molecules that can induce polarity in nonpolar waxes, enabling them to dissolve in cold water (a polar solvent). Mateu says that in the lab, he can make an emulsion with the cold process in about 20 minutes, as opposed to several hours of mixing, heating, and cooling with the conventional process. “Aesthetically, the product is the same thing—it feels the same and looks the same—so why not?” he says.
A short video demonstrating the cold-process formulation of a lotion with a Jeesperse emulsifier.
Home care
Many household cleaners and laundry detergents contain surfactants that emulsify oily dirt particles so that they can be diluted and washed away. Ethoxylated alcohols are a common ingredient of laundry detergents. Many detergents contain a blend of nonionic and anionic emulsifiers to lift stains out of textiles.
According to Sabatini, removing triglycerides such as fats, bacon grease, and vegetable oils from fabrics is particularly challenging. His lab has shown that extended surfactants, which are surfactants with intermediate polarity groups (e.g., polypropylene oxide and polyethylene oxide) inserted between the hydrophilic head and hydrophobic tail, are effective in removing these types of oily stains.
Industrial lubricants
Metalworking fluids and other industrial lubricants are typically o/w emulsions. Emulsifiers allow metalworkers to make use of both the lubricating properties of oils and the cooling capabilities of water. Anionic and nonionic emulsifiers are often used together in metalworking fluids. Cationic emulsifiers are rarely used because they are unstable in the alkaline solutions (pH 8–9.5) required for metalworking fluids.
Environmental technologies
Emulsions and microemulsions have been applied to environmental technologies such as subsurface remediation and biofuel production. For example, when oil or gas is spilled, the oil becomes trapped in pores in the soil and rock. Sabatini’s lab has developed alcohol-free microemulsions that help remove oil contaminants from the subsurface in an environmentally friendly manner. “The oil is trapped in the pores because of the interfacial tension between water and oil,” says Sabatini. “If we can lower that interfacial tension with emulsifiers, we can increase our rate of cleaning up contamination.”
In 1997, Sabatini and several colleagues founded a company called Surbec Environmental, LLC, to implement this technology. Since then, Surbec has assisted with the environmental cleanup of multiple sites in the United States and abroad. Examples include a gas station with a leaky underground tank and a military site contaminated with jet fuel.
Sabatini has also applied his emulsions research to the more efficient production of biofuel. Biodiesel is a vegetable oil, such as soybean oil, that has been chemically modified through a transesterification reaction to reduce its viscosity. “In terms of combustion, you don’t need to modify the vegetable oil. You can use vegetable oil in a diesel engine, and it’ll work pretty well without modification,” says Sabatini. “It’s just that vegetable oil has viscosity problems, especially at lower temperatures.”
As it turns out, microemulsification of vegetable oils can reduce viscosity without the need for the transesterification reaction. This would save time and allow more of the raw material to be used as fuel. However, Sabatini notes that the research is still in its early stages.
Although humans have been making emulsions for hundreds, if not thousands, of years, we are only now beginning to appreciate their diverse applications in many fields. Complex emulsions, such as microemulsions and multilayer emulsions, promise to further expand the repertoire of applications, particularly in emerging areas such as functional foods and biodiesel production. Now if only we could find an emulsifier for that difficult coworker.
Laura Cassiday is a freelance science writer and editor based in Hudson, Colorado, USA. She has a Ph.D. in biochemistry from the Mayo Graduate School and can be contacted at lauracassiday@yahoo.com.
Sidebar
What’s the difference?
The terms surfactant, emulsifier, and detergent are often used interchangeably, but there are distinctions.
Surfactant is the broadest term: Both emulsifiers and detergents are surfactants. Surfactants, or surface-active agents, are compounds that lower the surface tension between two liquids or between a liquid and a solid. Surfactants are amphiphilic, meaning that they contain hydrophilic (water-loving) head groups and hydrophobic (water-hating, or oil-loving) tails. Surfactants adsorb at the interface between oil and water, thereby decreasing the surface tension.
An emulsifier is a surfactant that stabilizes emulsions. Emulsifiers coat droplets within an emulsion and prevent them from coming together, or coalescing.
A detergent is a surfactant that has cleaning properties in dilute solutions.
Likewise, the terms emulsion, suspension, and foam are sometimes confused.
An emulsion is a mixture of two or more liquids, with or without an emulsifier, that are normally immiscible. One of the liquids, the “dispersed phase,” forms droplets in the other liquid, the “continuous phase.”
A suspension is a solid dispersed in a liquid. The particles are large enough for sedimentation.
A foam is a substance in which gas bubbles are suspended in a liquid.
Sidebar
Technical session highlights suspensions, emulsions, and foams
You can learn about the latest developments in suspensions, emulsions, and foams by attending a joint technical session on these topics at the upcoming 2014 AOCS Annual Meeting & Expo in San Antonio, Texas, USA. The session, which will be held on Wednesday, May 7, from 1:55–5 p.m., will feature a wide range of technical topics—from the fabrication of reduced-fat products by controlled aggregation of lipid droplets to the formulation of lipopeptide biosurfactant mixtures for dispersing oil spills in seawater.
The session is jointly sponsored by AOCS’ Edible Applications Technology (EAT) and Surfactant & Detergent (S&D) divisions, and is cross listed in the program as EAT 5.0 and S&D 5.1. A complete list of presentations.
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…