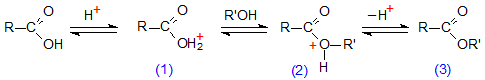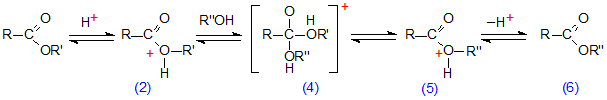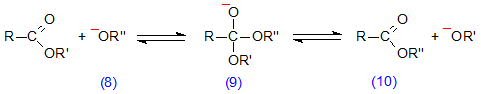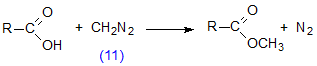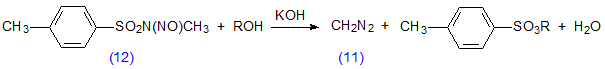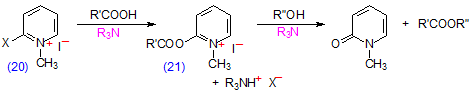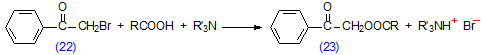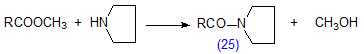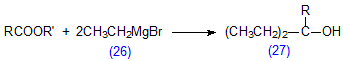The following was first published by W.W. Christie, in Advances in Lipid Methodology – Two, pp. 69-111 (1993) (Ed. W.W. Christie, Oily Press, Dundee), and it is reproduced here by kind permission of P.J. Barnes & Associates (The Oily Press), who retain the copyright. This review does not appear to have dated significantly and nothing equivalent has been published recently. A recent list of references to combined extraction/methylation can be accessed here, and some brief laboratory protocols are available on this site for methyl esters and for derivatives for mass spectrometry. There is also a ‘Beginners guide to the preparation of methyl esters’.
- Introduction
- Acid-Catalysed Esterification and Transesterification
1. General mechanism
2. Methanolic hydrogen chloride
3. Methanolic sulfuric acid
4. Boron trifluoride-methanol
5. Other acidic catalysts - Base-Catalysed Transesterification
1. General mechanism
2. Sodium and potassium methoxide catalysts
3. Organic base catalysis - Diazomethane and Related Reagents
1. Diazomethane and methyl ester preparation
2. Preparation of UV-absorbing and other derivatives - Pyrolysis of Tetramethylammonium Salts of Fatty acids for the Preparation of Methyl Esters
- Preparation of Esters and Amides via Activated Fatty Acids
1. Acid halides
2. Fatty acid anhydrides
3. Imidazolides
4. Other coupling reagents - Reaction of Alkyl or Aryl Halides with a Base
1. Derivatives for gas chromatography
2. Phenacyl esters and other derivatives for high-performance liquid chromatography - Alternative Methods for the Preparation of Esters, Amides and Other Fatty Acid Derivatives
1. Dimethylsulfate with dicyclohexylamine
2. Preparation of esters with alkyl formamide derivatives
3. Trimethylsilyl esters
4. Preparation of pyrrolidides from esters
5. Preparation of hydroxamic acid and related derivatives
6. Reaction with Grignard reagents - Special Cases
1. Short-chain fatty acids
2. Fatty acids with unusual structures
3. Sphingolipids and other N-acyl lipids
4. Sterol esters
5. Selective esterification of free fatty acids in the presence of other lipids - Preparation of Esters in the Presence of Adsorbents for Thin-Layer Chromatography
- Simultaneous Extraction from Tissues and Transesterification
- Artefacts of Esterification Procedures
- The Choice of Reagents – a Summary
- References
Introduction
The technique of gas chromatography (GC) revolutionized the study of lipids by making it possible to determine the complete fatty acid composition of a lipid in a very short time [53]. For this purpose, the fatty acid components of lipids are converted to the simplest convenient volatile derivative, usually methyl esters, although other esters may be preferred for specific purposes. The preparation of such esters has therefore become by far the most common type of chemical reaction for lipid analysts. On the other hand, there are other chromatographic techniques, notably high-performance liquid chromatography (HPLC) [52], where alternative derivatives, such as those with UV chromophores, are better. Picolinyl esters, 4,4-dimethyloxazolines or pyrrolidide derivatives have special properties for GC-mass spectrometry (MS) of fatty acids [128] (see also our mass spectrometry pages).
Although fatty acids can occur in nature in the free (unesterified) state, they are most often found as esters, linked to glycerol, cholesterol or long-chain aliphatic alcohols, and as amides in sphingolipids. The physical state of the lipids can vary. For example, they can be isolated as pure lipid classes or remain as a mixed lipid extract. It may be desirable to effect a reaction while lipids are still in the tissue matrix or on a chromatography adsorbent. There is no single esterification procedure that can be applied in all of these circumstances. The purpose of this review is to detail the principles behind the more important esterification and transesterification procedures, and to discuss their advantages and disadvantages and their applications to various classes of lipid. The various procedures are discussed with reference to the preparation of methyl esters from the more common C14 to C22 fatty acids mainly, in the free state or bound to lipids by ester or amide bonds. The preparation of esters from short-chain or unusual fatty acids is discussed in separate sections.
There have been a number of reviews of aspects of the topic [24,72,166,310], and the author has described esterification procedures for fatty acids on past occasions [49,52,53]; the latter reviews should be consulted for detailed recipes (as should my book co-authored with Xianlin Han – ‘Lipid Analysis’ – 4th edition’). The topic has also been reviewed from the standpoint of the synthetic organic chemist [129]. Many misconceptions continue to be published, and it is hoped that this survey of the methodology and discussion of the reaction mechanisms will help to eliminate these in future. The situation has not been helped by the publication of recommended methods by various societies, that may have been tested rigorously in quality control laboratories with gram quantities of seed oils, but are inappropriate for medical laboratories, say, where microgram amounts only of samples, containing high proportions of polyunsaturated fatty acids, may be available. In the latter circumstance, traces of water adsorbed to glassware, in solvents or in the atmosphere can be especially troublesome with many otherwise reliable procedures.
Acid-Catalysed Esterification and Transesterification
1. General mechanism
Carboxylic acids can be esterified by alcohols in the presence of a suitable acidic catalyst as illustrated in Scheme 1. The initial step is protonation of the acid to give an oxonium ion (1), which can undergo an exchange reaction with an alcohol to give the intermediate (2), and this in turn can lose a proton to become an ester (3). Each step in the process is reversible but in the presence of a large excess of the alcohol, the equilibrium point of the reaction is displaced so that esterification proceeds virtually to completion. However, in the presence of water, which is a stronger electron donor than are aliphatic alcohols, formation of the intermediate (2) is not favoured and esterification will not proceed fully.
Scheme 1. Acid-catalysed esterification of fatty acids.
Ester exchange or transesterification occurs under similar conditions (Scheme 2). In this instance, initial protonation of the ester is followed by addition of the exchanging alcohol to give the intermediate (4), which can be dissociated via the transitions state (5) to give the ester (6). Again, each step is reversible and in the presence of a large excess of the alcohol, the equilibrium point of the reaction is displaced so that the product is almost entirely the required ester (6). Water must once more be excluded, as it would produce some hydrolysis by dissociation of an intermediate analogous to (4) (R” = H) to a free acid. The preferred conditions for acid-catalysed esterification of carboxylic acids or transesterification of existing esters are therefore a large excess of the appropriate alcohol and absence of water. While it may be possible to obtain water-free conditions simply by adding anhydrous sodium sulfate to the reaction medium [256], a better practice in general is to operate with dry reagents and glassware.
Scheme 2. Acid-catalysed transesterification of lipids.
A critical practical point is the choice of acid as catalyst. This must facilitate the reaction but should not cause unwanted side effects. In principle, the methodology can be used with any alcohol component, but in practice it is limited to those alcohols that can be eliminated from the reaction medium by selective evaporation, i.e. methanol to perhaps pentanol.
2. Methanolic hydrogen chloride
The most frequently cited reagent for the preparation of methyl esters is 5% anhydrous hydrogen chloride (not hydrochloric acid) in methanol, prepared by bubbling dry gaseous hydrogen chloride into dry methanol. Gaseous hydrogen chloride is available commercially in cylinders or can be prepared when needed by dropping concentrated sulfuric acid onto fused ammonium chloride or into concentrated hydrochloric acid in a Kipp’s apparatus [100]. The stability of the reagent was studied by Kishimoto and Radin [183], who found that half the titratable acid was lost at room temperature in six weeks, presumably by reaction between the acid and methanol to give methyl chloride and water. Similar findings were obtained with 1-butanol-hydrogen chloride [125]. In practice, the small amount of water formed does not affect the esterifying reaction significantly and the reagent has a useful shelf life of about two weeks at room temperature or longer if refrigerated. An alternative method for rapid preparation of the reagent has been described [14] in which acetyl chloride is added to a large excess of dry methanol (Scheme 3). Methyl acetate is formed as a by-product but does not interfere with the reaction at the concentrations suggested; reagent prepared in this way is stable for about one week. It has been suggested that methanolic hydrogen chloride might be prepared by adding ammonium chloride and sulfuric acid to methanol [127].
Scheme 3. Preparation of methanolic hydrogen chloride via acetyl chloride.
In a typical esterification procedure using methanolic hydrogen chloride, the lipid sample is dissolved in at least a 100-fold excess of the reagent and the solution is refluxed for about two hours or is held at 50°C overnight (30 minutes at 50°C will suffice for free acids alone). At the end of this time, water is added and the required esters are extracted thoroughly into an appropriate solvent such as diethyl ether, hexane or light petroleum. The solvent layer is washed with dilute potassium bicarbonate solution to remove excess acid and dried over anhydrous sodium or magnesium sulfate (or anhydrous calcium chloride) and the esters are recovered after removal of the solvent by evaporation under reduced pressure on a rotary film evaporator or in a gentle stream of nitrogen. The reaction may also be performed in a sealed tube so that higher temperatures and shorter reaction times are possible. Longer reaction times are required as the molecular weight of the alcohol is increased.
All fatty acids are esterified at approximately the same rate by methanolic hydrogen chloride [126], so there are unlikely to be differential losses of specific fatty acids during the esterification step. On the other hand, special precautions are necessary to ensure quantitative recovery of short-chain esters and these are discussed in Section I.1. Certain classes of simple lipids, for example, cholesterol esters and triacylglycerols, are not soluble in methanolic hydrogen chloride alone and an inert solvent must be added to effect solution before the reaction will proceed. Benzene was once used frequently for the purpose, but the greater awareness of its toxicity now precludes its use. The author [49] has found that toluene, chloroform (ethanol-free), tetrahydrofuran and methyl-tert-butyl ether are all equally effective; the reaction is slower in methyl acetate, although this solvent has also been recommended [90].
While it has been claimed [163] that spurious components, which may interfere with GC analyses, are formed in hydrogen chloride-methanol solutions, these have not apparently been found by others. The author [49] has observed that such artefacts may be formed, apparently from the methanol, with a variety of acidic catalysts if superheating of the solution is allowed to occur in the presence of oxygen. With normal refluxing, under nitrogen especially, artefact formation is minimal.
Esterification will proceed with aqueous hydrochloric acid as catalyst if dimethoxypropane (7) is added to the reaction medium as a water scavenger (Scheme 4) [214]. Applications of this reagent to the esterification of free fatty acids [287] and transesterification of triacylglycerols [232,327] have been described. In a variation of the procedure, glycerol was determined at the same time as the methyl esters [231]. The method has a major disadvantage, however, in that large amounts of coloured polymeric by-products are formed from the dimethoxypropane [47,232,311,329], and these often interfere with the subsequent GC analysis of the methyl esters.
Scheme 4. Dimethoxypropane as a water scavenger.
Free fatty acids can be esterified with methanolic hydrogen chloride under mild conditions if they are first adsorbed onto a strongly basic anion-exchange resin [139,343]. In one procedure [139], other lipids were eluted from the column first with hexane, leaving the free acids behind for selective methylation; in another, it was demonstrated that it was even possible to methylate free acids in the presence of other lipids by related methodology (see Section I.5) [62]. Unfortunately, such procedures are time-consuming and tedious, especially as it is necessary to wash the resin first with large volumes of solvents to remove contaminants prior to the reaction [194]. In addition, recoveries of individual fatty acids were found to be uneven [139]. An alternative method for selective methylation of free acids in the presence of other lipids using methanolic HCl [206] required very precise timing and manipulation of the reactants and appears to have limited applicability.
Some evidence [140] has been presented that addition of cupric acetate to methanolic hydrogen chloride facilitates the esterification reaction, but the improvement appears to be marginal.
In summary, hydrogen chloride in methanol (or another alcohol) can be used to esterify free fatty acids or to transesterify fatty acids linked by ester bonds to glycerol or cholesterol. While it has been employed to transesterify amide-linked fatty acids, it is not the best reagent for this purpose (see Section I.3). It could probably be claimed to be the best general-purpose esterifying agent available. The main disadvantage is the comparatively long reflux time needed for complete reaction to be achieved. As with other acid catalysts, it is not suited for certain fatty acids with sensitive functional groups, such as epoxyl, cyclopropane or cyclopropene rings (see Section I.2).
3. Methanolic sulfuric acid
Although much higher concentrations are sometimes used, a solution of 1 to 2% concentrated sulfuric acid in methanol has almost identical properties to 5% methanolic hydrogen chloride, and is very easy to prepare. Indeed in one method [280,359,360], extraction of the tissue and esterification were carried out in isopropanol to which neat sulfuric acid was added (following removal of water from the extract) to catalyse formation of isopropyl esters for GC analysis (see also Section K). Esters of other alcohols, such as butanol [101], have been prepared similarly. The same reaction times are usually recommended for H2SO4– and HCl-methanol. Lie Ken Jie and Yan-Kit [208] showed that free acids were esterified especially rapidly with the former and a microwave oven as an energy source. Inert solvents must again be added to effect solution of simple lipids.
Free fatty acids were esterified very rapidly by heating in 10% sulfuric acid in methanol until the reflux temperature was reached [290], but this procedure cannot be recommended for polyunsaturated fatty acids, as sulfuric acid is a strong oxidizing agent. There are reports that very long reflux times (up to six hours) [124,174], excessive sulfuric acid concentrations (20%) [16] or high temperatures (170°C) [122,277] will lead to the formation of coloured by-products and the destruction of polyenoic fatty acids. With the dilute reagent and moderate temperatures, however, there is no evidence for side effects, and under such conditions the reagent was approved by the Instrumental Committee of the American Oil Chemists’ Society [10]. Of course, it has the same drawbacks as other acidic catalysts with sensitive fatty acids (see Section I.2).
McGinnis and Dugan [222] described a modification of the reaction in which the sulfuric acid complex of the lipid was formed in diethyl ether solution at −60°C first, before it was decomposed with anhydrous methanol to give the required methyl esters. The procedure was shown to be applicable to the direct methylation of lipids in biological materials (see Section K) [86]. Although the reaction is rapid, the practical manipulations required are complex and the method has rarely been used. Others have described the use of a pre-column, impregnated with sulfuric acid, at the head of a GC column, where the sample in methanol solution is esterified directly [171]. Such a technique would appear to be rather hazardous for the column packing!
4. Boron trifluoride-methanol
The Lewis acid, boron trifluoride, in the form of its coordination complex with methanol is a powerful acidic catalyst for the esterification of fatty acids. For example, esterification of free fatty acids was completed in two minutes with 12 to 14% boron trifluoride in methanol under reflux [236]. Morrison and Smith [259] showed that the reagent could be used to transesterify most lipid classes (inert solvent must again be added to effect solution of simple lipids), although in general longer reaction times are necessary than with free fatty acids. For example, in this reagent cholesterol esters are transesterified in 45 minutes at 100°C in a sealed Teflon™-lined screw-top tube. Boron trifluoride can of course be used with other alcohols, and as examples ethyl [164], propyl [297] and butyl [154,155,164] esters have been prepared in this way. Methyl esters labelled with tritium in the methyl group have been prepared by esterification of fatty acids with boron trifluoride and 3H-methanol [262]. The reaction has been accelerated by the use of microwave radiation (see also previous section) [23,73].
Unfortunately, boron trifluoride-methanol does have serious drawbacks. Lough [215] first reported that methoxy artefacts were produced from unsaturated fatty acids by addition of methanol across the double bond when very high concentrations of boron trifluoride in methanol (50%) were used. Although he later showed [216] that such artefacts were not necessarily formed with more normal concentrations of boron trifluoride in methanol (and this was confirmed by Morrison and Smith [259]), the warning was subsequently reiterated by others [65,97,185,190,235]. It is possible that the side reactions are exacerbated by the presence of oxidized lipids [65]. In addition, it has been reported that sample size is critical with substantial losses sometimes occurring with samples of less than 200 mg [314]. There is some evidence that artefact formation is most likely with aged reagents [97]. The author has had similar experiences in his own laboratory and has heard much anecdotal evidence in support. Although Klopfenstein [187] could not confirm the suggested explanations for artefact formation, he confirmed the existence of the problem. Boron trifluoride-methanol suffers from the same disadvantages as other acidic reagents with fatty acids with labile functional groups (see Section I.2 below), although there is a suggestion that it produces by-products quantitatively in some instances and that this may be of analytical value [185]. Troublesome by-products are also known to be formed from some antioxidants commonly added to lipid extracts (see Section L below).
Solutions of boron trifluoride in methanol obtained commercially should therefore be checked carefully before use and periodically in use. The reagent has a limited shelf life at room temperature and should be kept refrigerated. If such precautions are taken, the reagent may be a useful one in some circumstances. Problems are less likely to arise in quality control laboratories, say where there is a high throughput and large samples are the norm, than in medical laboratories where microgram amounts of lipids may be all that is available. The reagent has the blessing of the American Oil Chemists’ Society [11] and of IUPAC [151] amongst others. It is certainly highly popular, but possibly because it is one of the few such reagents that can be purchased from commercial suppliers. In view of the many known side reactions and the high acid content in comparison to other analogous reagents, this author would not consider using it in his own laboratory.
The reagent is of value for the oxidative fission of ozonides with simultaneous methylation in a method for double bond location in fatty acids [2,5].
Boron trichloride in methanol can be used in a similar manner to prepare methyl esters [1,38,39], although the reaction is slower than when boron trifluoride is the catalyst. Klopfenstein [187] established that artefact formation was much less of a problem with boron trichloride in methanol, and others [40] have shown that it does not cause disruption of cyclopropane fatty acids (see Section I.2).
5. Other acidic catalysts
Aluminium trichloride appeared to be as effective as boron trifluoride as a catalyst for transesterification [307], but it has not been tested with a wide range of samples. Phenyl esters of fatty acids have been prepared by acid-catalysed esterification with p-toluenesulfonic acid as catalyst [152]. Similarly, a strong cation-exchange resin (presumably with chemically-bonded phenylsulfonic acid moieties) in a fixed bed has been used as part of an HPLC system for post-column transesterification of lipids [294].
Base-Catalysed Transesterification
1. General mechanism
Esters, in the presence of base such as an alcoholate anion (8) (Scheme 5), form an anionic intermediate (9), which can dissociate back to the original ester or form the new ester (10). In the presence of a large excess of the alcohol from which the anion (8) was derived, the equilibrium point of the reaction will be displaced until virtually the sole product is the new ester (10). On the other hand, an unesterified fatty acid is converted to a carboxylate ion, RCOO–, in a basic solution, and this is not subject to nucleophilic attack by alcohols or bases derived from them because of its negative charge. Transesterification can therefore occur by this mechanism with basic catalysis but esterification cannot.
Scheme 5. Base-catalysed transesterification of lipids.
In the presence of water, the intermediate (9, R” = H) will dissociate irreversibly to the free acid. For base-catalysed transesterification of existing esters, it is necessary therefore to have a large excess of the new alcohol and an absence of water from the reaction medium.
2. Sodium and potassium methoxide catalysts
The most useful basic transesterifying agents are 0.5 to 2M sodium or potassium methoxide in anhydrous methanol, prepared by dissolving the clean metals in anhydrous methanol (the reaction is strongly exothermic). Potassium hydroxide at similar concentrations in methanol is occasionally used, but this is not recommended, since appreciable hydrolysis of lipids to free fatty acids can occur if the least trace of water is present, especially if the reaction is prolonged [107,142]. Methanolic sodium and potassium methoxide are stable for several months at refrigeration temperature but they eventually deteriorate, with precipitation of the bicarbonate salt by reaction with atmospheric carbon dioxide, and sometimes with the formation of other by-products, which may interfere in GC analyses. Artefact formation is minimized and the shelf life of the reagent improved if oxygen-free methanol is used in its preparation [49]. At equivalent molar concentrations with the same lipid samples, potassium methoxide effects complete esterification more quickly than does sodium methoxide, which is in turn more rapid than potassium hydroxide [49,218]. Because of the dangers inherent in handling metallic potassium, which has a very high heat of reaction with methanol, the author prefers to use sodium methoxide in methanol in his own laboratory. It should be noted that, in common with all strongly alkaline solutions, these reagents should be handled with caution.
Sodium methoxide in methanol effects transesterification of glycerolipids much more rapidly than is sometimes realized, and although reflux times of as long as 6 hours have on occasion been recommended in the literature, it has been shown that triacylglycerols can be completely transesterified in 2 to 5 minutes and phosphatidylcholine in only 1 minute at room temperature [226,227]. The reaction is slower with alcohols of higher molecular weight, i.e. taking up to 60 minutes with hexanol [102]. As with acidic catalysis, inert solvents must be added to dissolve simple lipids before methanolysis will proceed. Benzene has been used frequently for the purpose, but is no longer recommended for safety reasons, and the author [49] has found that the reaction is as fast or faster with dry toluene, dichloromethane (not with phospholipids), methyl-tert-butyl ether and tetrahydrofuran, and a little slower in diethyl ether, hexane or dimethyl carbonate. Early reports that this last solvent accelerates transesterification by participation in the reaction have now been refuted [59]. While chloroform is frequently recommended as a suitable solvent, the author [49] has noted that it reacts with sodium methoxide to give a precipitate of sodium chloride, presumably with generation of dichlorocarbene, which has the potential to react with the double bonds of unsaturated fatty acids (although this does not appear to have been demonstrated in practice) [178]. Acetone is unsuitable also.
In a typical transesterification reaction, the lipid sample, dissolved if necessary in sufficient toluene or other solvent to ensure it remains in solution, is reacted with a 100-fold excess of 0.5 to 2M sodium methoxide at 50°C (refluxing will speed up the reaction but is not usually necessary). Triacylglycerols are completely transesterified in 10 minutes and phosphoglycerides in 5 minutes, although cholesterol esters require 60 minutes, under these conditions. At the end of the appropriate time, dilute acid is added to neutralize the sodium methoxide and so minimize the risk of hydrolysis occurring, and the required methyl esters are recovered by solvent extraction as with acid-catalysis. As an alternative for the micro-scale, a method can be recommended in which the reaction is carried out in an inert solvent with the minimum of methanolic sodium methoxide and with methyl acetate added to suppress the competing hydrolysis reaction [51].
A novel technique was described by Dutton and colleagues [34,74] in which methylation was carried out in a micro-reactor at the head of a GC column immediately prior to analysis. A heated sodium hydroxide-impregnated pre-column has also been used [172]. DePalma [76] adapted a laboratory robotic system for a similar purpose, while others have described the use of potassium methoxide/celite in small columns for methylation [228,304]. Recently, alumina treated with methanol has been incorporated into a supercritical fluid extraction-chromatography system to effect methylation on-line [181].
A reagent consisting of sodium borohydride with sodium hydroxide in methanol has been suggested as a means of reducing hydroperoxides to hydroxides and minimising over-oxidation during trans-esterification [283,284]. Hydroperoxides per se are not conveniently or safely methylated by any of the usual procedures (see Section I.2) [338].
Sodium methoxide in methanol is therefore a valuable reagent for rapid transesterification of fatty acids linked by ester bonds to alcohols (e.g. cholesterol, glycerol). It will not esterify free fatty acids or transesterify amide-bound fatty acids in sphingolipids. Also, unlike acidic catalysts, it will not liberate aldehydes from plasmalogens. Under normal conditions, no isomerization of double bonds in polyunsaturated fatty acids occurs [102,157], but prolonged or careless use of basic reagents can cause some alterations of fatty acids [17]. As examples, ethyl [102,108,164], propyl [102,109,122], butyl [102,109,298], isobutyl [328], isopentyl [328], hexyl [102] and phenylethyl [56] esters have been prepared by reaction with sodium in the appropriate alcohol.
3. Organic base catalysis
Pyrolysis of quaternary ammonium salts of free fatty acids was used as a method of methylation for some years (see Section E below), before it was realized that quaternary ammonium hydroxides could catalyse transesterification of lipids as part of relatively simple procedures for preparing methyl esters for GC analysis. The first use of such a procedure was in fact a quaternary ammonium ion exchange resin in the methoxide form [77], that was reported to be particularly suitable for transesterification of oils containing hydroxy fatty acids with conjugated double bond systems (dimorphecolic and lesquerolic acids) (see also Section I.2). However, the first reagent suited to use in homogeneous solution to be described was 0.2M methanolic (m-trifluoromethylphenyl)trimethylammonium hydroxide [219]. The glyceride sample in benzene was converted to the methyl esters in 30 minutes at room temperature, and it was possible to inject an aliquot of the reaction mixture directly onto the GC column without further work-up. If any free acids were present, they were converted to methyl esters in the heated injection port. A minor drawback is that N,N-dimethyl(m-trifluoromethyl)aniline, which is formed as a by-product, elutes as a distinct peak after the solvent front. The same type of reagent has been used by others to produce methyl [61,338], benzyl [337] and pentafluorobenzyl [291,339] esters from a variety of different lipid classes and fatty acids, including those derived from hydroperoxides. Tetramethylammonium hydroxide has been used as catalyst in a similar way [89,230,237,248] as has tetramethylguanidine hydroxide [302].
The most useful reagent of this type now appears to be trimethylsulfonium hydroxide ((CH3)3SOH), prepared ideally under conditions that guarantee the elimination of water [43,44,92], since it reacts very rapidly with lipids and the only by-product, dimethyl sulfide, is highly volatile [43,44,367] (see also Section E below). It was observed [92] that triacylglycerols and phospholipids were transesterified at a rate only a little slower than with sodium methoxide, but cholesterol esters were methylated very slowly. Polyunsaturated fatty acids, such as those of fish oils, were methylated safely. Although the reagent has seen limited use so far [233,264,265,303], it obviously has great potential.
Diazomethane and Related Reagents
1. Diazomethane and methyl ester preparation
Diazomethane (11) reacts rapidly with unesterified fatty acids to give methyl esters (Scheme 6), but does not effect transesterification of other lipids (see also Section I.5 below). The reaction is not instantaneous, however, as has sometimes been assumed, unless a little methanol is present as a catalyst [70,110,301]. The reagent is generally prepared in ethereal solution by the action of alkali on a nitrosamide, e.g. N-methyl-N-nitroso-p-toluene-sulfonamide (12) (Diazald™, Aldrich Chemical Co., Milwaukee, U.S.A.) in the presence of an alcohol (Scheme 7). A large-scale practical procedure has been described [35], but simple small-scale preparations, in which special glassware is used and only enough diazomethane for immediate needs is prepared, are to be preferred [67,207,301,346]. An alternative procedure involving the reaction of hydrazine hydrate, chloroform and alkali has also been recommended [67]. Diazomethane has been used to prepare methyl esters labelled with 14C [301,318] or tritium [189] in the methyl group.
Scheme 6. Reaction of diazomethane with a fatty acid.
Scheme 7. Preparation of diazomethane from N-methyl-N-nitroso-p-toluenesulfonamide (12) (Diazald™).
Solutions of diazomethane in diethyl ether (with a little alcohol) are stable for short periods if stored in the dark over potassium hydroxide pellets at refrigeration temperatures. If they are kept too long, polymeric by-products are formed which interfere with GC analyses [122,258,301]. It has been claimed that loss of polyunsaturated fatty acids will occur by addition of carbene, formed by decomposition of diazomethane, to double bonds [122] or by related mechanisms [329]. Although others [301] were unable to detect this effect with a conventional range of fatty acids, it was found with α,β-unsaturated and α-keto acids [31]. There have been reports of etherification of hydroxy fatty acids with diazomethane, either with the absence of a small amount of methanol from the medium or in the presence of acidic or basic catalysts when reaction was prolonged [110,137], but this does not appear to be a problem otherwise. There is also evidence that diazomethane can cause some transesterification by prolonged irradiation under UV light in the presence of traces of acidic or basic catalysts [271].
Diazomethane is potentially explosive and great care must be exercised in its preparation; in particular, apparatus with ground-glass joints and strong light must be avoided. The reagent is toxic and is liable to cause development of specific sensitivity; nitrosamides used in the preparation of diazomethane must be handled with care, as they are potential carcinogens. Small-scale procedures are recommended [67,207,301,346], since if sensible precautions are taken, the risks to health are slight, while methyl esters are prepared rapidly with virtually no artefact formation. Suitable micro-equipment is available from commercial sources.
2. Preparation of UV-absorbing and other derivatives
Pentafluorodiazoalkanes have been used to prepare derivatives from unesterified fatty acids that are suitable for GC with sensitive electron-capture detection [135], and benzyldiazomethane has been used to prepare benzyl esters [132,186].
Related methods have been used to prepare UV-absorbing derivatives for analysis by HPLC. For example, 9-anthryldiazomethane in diethyl ether, methanol, ethyl acetate or acetone solution gave good yields of anthrylmethyl esters in some hands [26,147,268,366], but others have preferred alternative methods (see Section G.2). 1-Pyrenyl-diazomethane has been used also for derivative preparation [269].
Pyrolysis of Tetramethylammonium Salts of Fatty Acids for the Preparation of Methyl Esters
Robb and Westbrook [289] first showed that tetramethylammonium salts of unesterified fatty acids in aqueous solution could be pyrolysed to form methyl esters in the heated (330 to 365°C) injection port of a gas chromatograph, but they were unable to obtain quantitative recovery of individual components of mixtures. However, by drying the samples carefully prior to analysis and using solid injection into the gas chromatograph [82,83] or by improvements in the design of the injector [21], others were able to obtain quantitative yields of methyl esters. The former adaptation was shown to be especially suited to the preparation of methyl esters from the mono- and dibasic acids obtained by oxidative cleavage of unsaturated fatty acids [84]. Although the method was used initially with saturated components, unsaturated fatty acids could also be esterified if the reaction conditions were controlled rigorously [83].
Subsequently greatly improved results were obtained by changing to trimethyl(m-trifluoro-methylphenyl)ammonium hydroxide [105,184,220] or trimethylphenylammonium hydroxide [41,353] as the catalyst, when even polyunsaturated fatty acids in the free form could be analysed successfully.
Trimethylsulfonium and trialkylselenonium hydroxides could be even better, since they were found to decompose at lower temperatures (approximately 200°C) and the by-products produced (dimethyl sulfide from the former as illustrated in Scheme 8) are relatively volatile and do not interfere with the GC analysis [44,367]. However, some loss of polyunsaturated fatty acids was found with trimethylsulfonium hydroxide [92]. In some of these applications, samples were hydrolysed to the free acids prior to esterification, although it is now realized that the same catalysts can effect transesterification directly (see Section C.3 above).
Scheme 8. Methylation of fatty acids by pyrolysis of the salt formed from trimethylsulfonium hydroxide (TMSH).
The method may be of value for the analysis of unesterified fatty acids in aqueous solution as there is no extraction step during which selective losses of specific components may occur. It also appears to have value for selective esterification of free acids in the presence of other lipids (see Section I.5). On the other hand, this approach cannot be used with on-column injection techniques for gas chromatography.
Preparation of Esters and Amides via Activated Fatty Acids
1. Acid halides
Acid chlorides and anhydrides react with alcohols to give esters and with amines to give amides under appropriate conditions. The preparation and properties of acid chlorides and anhydrides have been reviewed [112]. Activation of fatty acids in this way is of special utility in the synthesis of esters of glycerol, for example for the synthesis of triacylglycerols or phosphoglycerides with specific fatty acids in the various positions [117]. More relevant to the topic of this review, such methodology can be of value for the synthesis of derivatives where the alcohol component has a relatively high molecular weight (six carbon atoms or more) for chromatographic analysis. Methods of this kind are preferred for the preparation of simple amine derivatives, but they do not have any advantages over other procedures described here for preparation of esters from short-chain aliphatic alcohols.
The use of acid chlorides for glycerolipid synthesis was reviewed by Mattson and Volpenhein [Mattson, F.H. and Volpenhein, R.A. J. Lipid Res., 3, 281-296 (1962)]. The mildest method of preparation is probably to react the acids with oxalyl chloride; the reaction is slow (up to 3 days at room temperature) but chlorides of polyunsaturated fatty acids can be prepared safely. Thionyl chloride will react much more quickly, and though it can cause isomerization of double bonds if used carelessly, this is not a problem if the reaction time is confined to 1 minute [128]. In addition, fatty acid chlorides have been prepared under gentle conditions by reacting an acid with triphenylphosphine and carbon tetrachloride [134].
In practice to prepare an ester, the acid chloride is reacted with the required alcohol under strictly anhydrous conditions in the presence of a basic catalyst, traditionally pyridine or triethylamine but more often N,N‘-dimethyl-4-aminopyridine or 4-pyrrolidinopyridine nowadays, to mop up the acid produced (Scheme 9). As an example, L-menthyl esters were prepared by reacting menthol with the appropriate acid chloride [3,4], in order to resolve optically active fatty acids by GC. Acid chlorides prepared by a rapid reaction in situ with thionyl chloride were favoured by Harvey [128] for the preparation of picolinyl (3-hydroxymethylpyridinyl) esters of fatty acids for analysis by GC/MS.
Scheme 9. Preparation of esters via acid chlorides.
Similar methodology has been used for the synthesis of simple amide derivatives, such as those of p-methoxyaniline [134], naphthylamine [148], 9-aminophenanthrene [149] and aniline [197].
2. Fatty acid anhydrides
Fatty acid anhydrides, when used in the same way as acid chlorides, are reported to give fewer by-products. They have often been used with the tetraethylammonium salt of the same acid in the synthesis of lipids per se [68,200,201], but mixed acid anhydrides of fatty acids and trifluoroacetic acid are much more useful for the preparation of derivatives for chromatography [54,58,113,272]. With the latter, the mixed anhydride is prepared by reaction with trifluoroacetic anhydride immediately before use. Heptafluorobutyric anhydride has been utilized in a similar manner to prepare trichloroethyl esters [323]. While the presence of a base, such as N,N-dimethyl-4-aminopyridine, to neutralize the acid by-product is not essential, it appears to improve the yield when the reaction is carried out on the sub-milligram scale [54,58].
3. Imidazolides
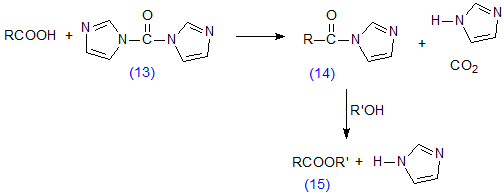
Apart from the fact that preparation of esters via acid chlorides and anhydrides involves two steps, such procedures suffer from the disadvantages that they cannot be used for fatty acids with acid-labile functional moieties, e.g. epoxyl groups, and they tend to give poor yields with small samples (less than 1 milligram) when the slightest trace of atmospheric moisture is present, a situation encountered commonly in chromatographic analysis. A useful alternative, which gives high yields in terms of the fatty acid components [37,114], is then to activate the latter by reacting them with 1,1′-carbonyldiimidazole (13) to form an imidazolide (14), which is reacted immediately (without isolation) with the alcohol in the presence of a base to give the required ester (15) (Scheme 10). This procedure has been used in the preparation of picolinyl ester derivatives from epoxy [22] and thia [57] fatty acids, for example. It has also been employed for selective methylation of the free fatty acid fraction from plasma in the presence of other lipids (see also Section I.5) [188]. 1,1′-Carbonyldiimidazole is very sensitive to moisture and must be stored in a desiccator.
Scheme 10. Preparation of esters via imidazolide derivatives of fatty acids.
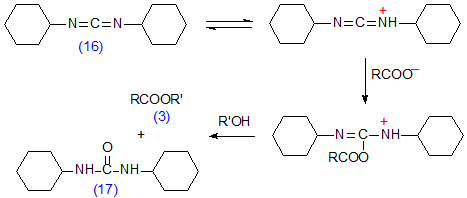
Dicyclohexylcarbodiimide (16) is a related reagent for effecting the coupling of a fatty acid and alcohol as shown in Scheme 11. It gives quantitative esterification even in the presence of moisture, a considerable virtue when sub-milligram quantities of fatty acids must be derivatized. However, some drawbacks have been reported [129]. The N,N-dicyclohexylurea (17), produced as a by-product, is not easily removed from the reaction medium, and the reagent is potentially carcinogenic and must be handled with great care. The reaction has been used most often for the preparation of synthetic glycerolipids [117] rather than for simple derivatives, but it has been employed for the synthesis of dansyl-ethanolamine derivatives of fatty acids [293].
Scheme 11. Preparation of esters with dicyclohexylcarbodiimide (16) as the coupling agent.
2-Nitrophenylhydrazide derivatives of long-chain fatty acids have been prepared by coupling the free acids with 2-nitrophenylhydrazine hydrochloride in the presence of dicyclohexylcarbodiimide or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride [249-254]. The same type of reaction was used as part of a post-column HPLC detection system to prepare fluorescent hydrazine derivatives for HPLC [156].
4. Other coupling reagents
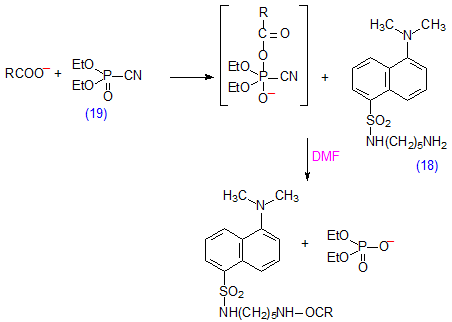
Amide derivatives of monodansylcadaverine (18) have been prepared by coupling the amine and fatty acids in the presence of diethylphosphorocyanidate (19) (Scheme 12) [202,203]. The reaction occurred rapidly under simple practical conditions, but the coupling reagent is not yet readily available from commercial sources.
Scheme 12. Preparation of esters with diethylphosphorocyanidate (19) as the coupling agent.
An alternative method consists in reacting the fatty acid with the appropriate alcohol in the presence of 2-bromo-1-methylpyridinium iodide (20) as catalyst (Scheme 13) [296]. The reaction proceeds via an intermediate 2-acyloxy-1-methylpyridinium iodide (21), which is subjected to nucleophilic attack by the alcohol in the presence of a tertiary amine to mop up the hydrogen iodide produced. As activation of the fatty acid and esterification takes place in one step, there are obvious advantages, which should be explored further. The procedure was used for the preparation of anthrylmethyl esters of fatty acids, where it was reportedly superior to the use of anthryldiazomethane (see Section D.2 above) for the purpose [28-30,211]. 2-Chloro-1-methylpyridinium iodide has been used similarly to prepare N-(4-aminobutyl)-N-ethyl-isoluminol esters for HPLC with fluorescence detection [368].
Scheme 13. Preparation of esters with 2-bromo-1-methylpyridinium iodide (20) as the coupling agent.
Reation of Alkyl or Aryl Halides with a Base
1. Derivatives for gas chromatography
Methyl esters can be prepared quantitatively by reaction of the silver salts of fatty acids with methyl iodide [104]. Although the reaction was said to be especially suitable for samples containing short-chain fatty acids [104,161], and an application to the analysis of milk fats was described, better methods are now available (see Section I.1). The method was subsequently applied to the preparation of benzyl esters, but a number of by-products were formed which interfered with the reaction [160].
In essence, the reaction involves SN2 attack of a basic anion on an alkyl halide in a highly polar solvent system such as dimethylformamide (see Scheme 14 below). Other basic catalysts have been employed for the preparation of methyl esters including the organic catalysts phenyltrimethylammonium hydroxide [111] and tetramethylammonium hydroxide [121,351], sodium and potassium hydroxide [63,162], potassium carbonate [115], potassium hydroxide [79] and potassium ion-crown ether complexes (see next section) [133]. Similarly, benzyl esters have been prepared by reaction of potassium carboxylates with benzyl bromide and catalysed by a crown ether in acetonitrile solution [300]. Analogous methods have been used to prepare pentafluorobenzyl esters, i.e. by reacting the free acid with pentafluorobenzylbromide and triethylamine in acetonitrile solution, for analysis by electron capture GC [71,120,223,315,362]. The reaction has not been widely used but may be a useful alternative to diazomethane for the preparation of esters from free acids in the presence of other lipids.
A related method has been used to prepare methyl esters from the fatty acids of sphingolipids [162]. In this instance, the hydroxyl groups of the 2-hydroxy fatty acids present were methylated simultaneously (see also Section I.3 below) [63,162].
2. Phenacyl esters and other derivatives for high-performance liquid chromatography
Phenacyl esters and related derivatives, which absorb strongly in the UV region of the spectrum, are of particular value for analysis by means of HPLC. The method of preparation is in essence identical to that described above, but the reaction must be optimized for minimum use of the reagents, partly because of cost and partly because of the difficulty of removing the excess from the medium. Crown ethers, i.e. cyclic polyethers such as 1,2,7,10,13,16-hexaoxacyclooctadecane (“18-crown-6”), complex strongly with potassium ions and effectively solubilize them in aprotic solvents such as toluene, acetonitrile, methylene chloride and carbon tetrachloride, thus facilitating the reaction and simplifying the isolation of the esters produced. For example, phenacyl esters have been prepared by reaction of phenacyl bromide and a fatty acid in the presence of potassium carbonate and a crown ether in acetonitrile solution [88]. As an alternative, phenacyl esters (23) can be prepared under mild conditions by reaction of phenacyl bromide (22) and a fatty acid in the presence of triethylamine in acetone solution (Scheme 14) [36,361]. The procedure of Wood and Lee [361] can be recommended. Some stereomutation of double bonds in phenacyl esters was observed when attempts were made to purify them by TLC [361]
Scheme 14. Preparation of phenacyl esters of fatty acids.
Methoxyphenacyl [242], bromophenacyl [165], naphthacyl [64,79,165], methylanthryl [191], methylisatin [116], 4-bromomethyl-7-methoxycoumarin [87,198,357,358], 4-bromomethyl-7-acetoxy-coumarin [171,330-332] and other [267,364,365] esters have been prepared in the same way. The list is by no means complete. p-Nitrobenzyl and other UV-absorbing derivatives of fatty acids were prepared in an analogous manner by reaction with the p-toluenesulfonate derivatives of the appropriate alcohols in the presence of a basic catalyst [98], and bromophenacyl esters have been prepared via trifluoromethanesulfonates [150].
An alternative technique for solubilizing an anion for reactions of this type is to use a phase-transfer-mediated reaction catalysed by tetrabutylammonium ions, which can at the same time extract the fatty acids from an aqueous medium. Thus, free fatty acids have been extracted and simultaneously converted to phenacyl esters by this means [99,369], and methyl, 4-nitrobenzyl [244,245], anthracyl, acetylfluorene [8], methylacridine [336] and N-(9-acridinyl)-acetamido [9] esters have been prepared similarly.
Alternative Methods for the Preparation of Esters, Amides and Other Fatty Acid Derivatives
1. Dimethylsulfate with dicyclohexylamine
Methyl esters can be prepared from unesterified fatty acids in a basic medium by heating them with dimethylsulfate in the presence of dicyclohexylamine for 15 to 60 minutes [317]. The method may be useful for esterifying fatty acids where diazomethane is not suitable or for fatty acids with functional groups that might be altered under acidic conditions. Unfortunately, the procedure does not appear to have been tested with a wide range of different fatty acids. Ethyl esters can be prepared in a similar way.
2. Preparation of esters with alkyl formamide derivatives
Carboxylic acids react with alkyl formates in dimethylformamide solution to give esters in good yield, and the method was used to prepare a variety of different esters [325]. In practice, alkyl chloroformates (24) are now the preferred reagents since they react very rapidly with acids under mild conditions in the presence of N,N-dimethyl-4-aminopyridine (Scheme 15) [180]. Others carried out the reaction in a solution of acetonitrile, the alcohol and pyridine [144,146], though other basic catalysts, such as 3-picoline, N-methylpiperidine and dimethylaminopyridine appeared to be preferable [344]. When the method was applied to 2-hydroxy fatty acids, simultaneous alkylation of the hydroxyl group occurred [144]; this did not happen when the hydroxyl group was in other positions. The reaction was found to be less sensitive to traces of moisture than is the case in many other procedures [146,180,344]. As it is carried out in a mildly basic medium and is very rapid, the procedure may repay further study for esterification of fatty acids with labile functional groups. The use of chloroformates for derivatization has been reviewed by Husek [145].
Scheme 15. Preparation of esters by reaction of acids with chloroformates.
3. Trimethylsilyl esters
Trimethylsilyl esters of fatty acids have been prepared for GC analysis, but they do not appear to be as useful as the conventional alkyl esters for the purpose, as they are sensitive to the presence of traces of water. In addition, non-polar silicone liquid phases must be used in the GC columns and these tend to have poorer resolving powers than the more usual polyesters. The common silylating agents, hexamethyldisilazane and trichloromethylsilane for example, can be used for the preparation of trimethylsilyl esters [60,81,94,173,196,229]. Woollard [362] has investigated procedures for preparing tert-butyldimethylsilyl esters of fatty acids.
4. Preparation of pyrrolidides from esters
Pyrrolidide derivatives of fatty acids are of value for structural identification of fatty acids by GC/MS [12,128]. Strongly nucleophilic bases such as pyrrolidine will react with esters (methyl, glyceryl, etc.) in the presence of acetic acid at 100°C (for 1 hour) to form pyrrolidides (25) (Scheme 16) [13]. However, the reaction has been accelerated appreciably by the use of microwave radiation [73]. In addition, pyrrolidides have been prepared from free fatty acids via trimethylsilyl esters in a one-pot reaction [340] (see also our specialist web page on this topic).
Scheme 16. Preparation of pyrrolidide derivatives from methyl esters.
4,4-Dimethyloxazoline derivatives also have useful properties in locating functional groups by MS, but quantitative preparation requires heating the methyl esters with 2-amino-2-methylpropanol at 180°C overnight [95].
5. Preparation of hydroxamic acid and related derivatives
Hydroxamic acid derivatives of fatty acids have been prepared for HPLC analysis by the nickel-catalysed reaction of a fatty acid and hydroxylamine hydrochloride [123] and by reacting the fatty acid with hydroxylamine perchlorate [119]. Isopropylidene hydrazides have been prepared by hydrazinolysis followed by acetonation [6].
Imidazole derivatives of fatty acids for HPLC analysis were synthesised by reaction with 9,10-diaminophenanthrene, but strenuous reaction conditions were required [212].
6. Reaction with Grignard reagents
Fatty acid esters react with Grignard reagents, such as ethyl magnesium bromide (26), to form tertiary alcohols (27) (Scheme 17). The reaction was first used to convert the fatty acid components of the wax esters from jojoba oil to tertiary alcohols, the primary alcohols being released as such [282]. The two groups of compounds could be analysed simultaneously by GC in this form. More recently, it has been shown that the reaction can be used with a variety of fats and oils, especially those containing short-chain fatty acids as in milk fat [281]. Different alkyl moieties were employed to give theoretical response factors for flame-ionization detection that were close to unity, even with butyric acid. Although the reaction products are very different from the esters that have traditionally been favoured for GC analysis, the methodology is simple and may have wide applicability; it might repay further study.
Scheme 17. Reaction of fatty acids with a Grignard reagent, ethyl magnesium bromide (26).
Special Cases
1. Short-chain fatty acids
Much attention has been given to the preparation and analysis of esters of short-chain fatty acids by GC, largely because of their occurrence in milk fats. Reviews of the problems in such analyses have been published [53,158]. Short-chain fatty acids, in the free state or esterified to glycerol, can be converted completely to methyl esters by any of the reagents described above, but quantitative recovery from the reaction medium may not be achieved unless special precautions are taken. Losses can occur at several stages in any procedure. Short-chain fatty acid esters (methyl especially) are volatile and may be lost selectively on refluxing the esterification medium, they are more soluble in water than longer-chain esters and can be lost in an aqueous extraction step, or they may be distilled off when the extracting solvent is evaporated. Selective losses can also occur if non-saponifiable impurities have to be removed by sublimation or thin-layer chromatography (TLC) purification.
Losses occurring during refluxing of solutions can be avoided by carrying out the reaction in a sealed vessel or using procedures that work satisfactorily at room temperature. Losses of short-chain esters during aqueous extractions can never be eliminated entirely, but they can be kept to a minimum with care. Some of the factors involved were studied by Dill [78], who found that recoveries could be improved greatly by salting out the esters. Hydrocarbon solvents, such as hexane, pentane or light petroleum gave better recoveries than did diethyl ether or benzene. Others [174] obtained excellent recoveries of short-chain esters by extracting with a solvent mixture of docosane and petroleum ether, but the docosane could interfere with the subsequent GC analysis of the esters. Careful removal of excess solvents at low temperatures on a rotary film evaporator is better than using a stream of nitrogen and will keep losses of short-chain esters down, but these cannot be eliminated completely.
The best esterification procedures for short-chain fatty acids are those in which heating of the reagents is avoided and in which stages involving aqueous extraction and solvent removal are absent. Many analysts, including the author, have found that the alkaline transesterification procedures of Christopherson and Glass [59] and of Luddy et al. [217] are the most convenient and quantitative for obtaining short-chain methyl esters from lipids, as no aqueous extraction or solvent removal steps are necessary. In the latter procedure, the lipid sample was transesterified at 65°C with a small amount of 0.4M potassium methoxide in a sealed tube. On cooling, carbon disulfide was added and excess methanol was removed by adding anhydrous calcium chloride. An aliquot of the supernatant solution was injected directly into the gas chromatograph. If the sample contained a high proportion of free fatty acids, these were esterified with boron trifluoride in methanol after the transesterification reaction had been completed. In the method of Christopherson and Glass [59], the fat sample was dissolved in 19 volumes of petroleum ether in a stoppered-tube and one volume of 2M sodium methoxide in methanol was added. Transesterification was complete in a few minutes at room temperature and an aliquot of the reaction mixture was again injected directly onto the GC column. If appreciable amounts of free fatty acids were present in the sample, they could be methylated when the transesterification reaction was finished by adding 5 volumes of 10% hydrogen chloride in methanol and leaving the reaction mixture for a further hour at room temperature before injection onto a packed GC column.
Injection of reaction media containing basic and acidic esterification catalysts directly onto GC columns shortens their working lives. The top few centimetres of packed columns can be replenished periodically, while lengths of deactivated tubing or “retention gaps” ahead of capillary columns will protect the latter. This can be a price some wish to pay for the speed, simplicity and accuracy of these procedures.
The method of Christopherson and Glass has been evaluated and recommended by Badings and De Jong [20]. Others have suggested minor modifications in which acid was added to neutralise the sample prior to GC analysis [25,53] or in which methyl acetate was added to suppress the competing hydrolysis reaction [51,53]. Basic transesterification-esterification with a quaternary ammonium salt has also been considered [230]. Of course, it is necessary that the GC conditions be optimized for the analysis otherwise the value of these transesterification procedures will be negated [20,25,53].
Quantitative recovery of esters of short-chain acids is less of a problem if esters of higher molecular weight alcohols than methanol are prepared. For example, propanol, butanol or 2-chloroethanol may be used, but great care is still necessary. For example, a procedure utilizing boron trifluoride-butanol has been recommended [154,155] and the Grignard reaction described above (Section H.6) appears to have some promise [281]. In addition to the combined methods just described, free short-chain fatty acids can be esterified with diazomethane in diethyl ether and the reaction medium injected directly onto the GC column. Alternatively, the use of alkyl chloroformates could be considered (Section H.2). Methods requiring hydrolysis prior to esterification are not recommended.
HPLC methods have also been developed for the analysis of milk fatty acids. Benzyl esters of milk fat were prepared by transesterification with potassium benzylate [55], and butyrate in milk fat was determined in the form of the phenethyl ester [56]. As an alternative, milk fat was saponified with potassium hydroxide, solvent was removed from the alkaline solution and the potassium salts were solubilized with a crown ether for conversion to phenacyl or related esters [179]. A similar method was described for the analysis of the free fatty acid fraction in butter fat [288].
2. Fatty acids with unusual structures
If the appropriate precautions are taken, most of the methods described above can be used to esterify unsaturated fatty acids with two to six methylene-interrupted double bonds without causing stereomutation or double bond migration, i.e. they can be safely applied to virtually all fatty acids of animal origin with the possible exception of certain of the eicosanoids. Plant lipids, on the other hand, may contain fatty acids with a variety of different functional moieties, such as cyclopropene rings, conjugated unsaturation (especially of concern when adjacent to other functional groups), or epoxyl groups, which can be altered chemically by certain esterification catalysts [19,312]. Some knowledge of the chemistry and potential occurrence of these fatty acids is necessary, therefore, before a decision can be taken as to which of the available methods of esterification is likely to be most appropriate in each instance.
The occurrence and chemistry of cyclopropene and cyclopropane fatty acids have been reviewed [48,306]. Both types of functional group may be destroyed by acidic conditions, but triacylglycerols and other lipids containing such acids can be transesterified safely with basic reagents. Unesterified fatty acids can be methylated with diazomethane and presumably by alkyl chloroformates (Section H.2) or via the imidazolides (Section F.3). Boron trifluoride-methanol complex, although it is acidic, was reportedly suitable for esterifying cyclopropene fatty acids without causing any alteration to the functional group [185]. However, this reagent reacts with cyclopropane fatty acids, which are often found with cyclopropene fatty acids in seed oils, with the addition of methanol across the ring carbons [75,243,247], a property that can be of diagnostic value [243,247]. Methanolic-hydrogen chloride has similar drawbacks [199,345], but boron trichloride-methanol has been used safely [40].
Epoxy fatty acids are widely distributed in seed oils, and their occurrence and chemical reactivity have been reviewed [193]. Fatty acids of this type are very sensitive to acidic conditions and they react with opening of the oxirane ring. For example, hydrogen chloride adds across the ring to form halogen hydrins, and boron trifluoride-methanol adds methanol across the ring to give a methoxy-hydroxy product that is potentially useful for quantitative analysis of epoxy fatty acids in natural oils [185]. Epoxy acids are not harmed by basic conditions under normal circumstances, however, and seed oils containing these acids can be transesterified safely with alkaline reagents. Unesterified epoxy acids can be methylated with diazomethane and they have been converted to picolinyl esters via the imidazolides (Section F.3) [22].
The occurrence and chemistry of fatty acids with conjugated unsaturation have been reviewed [138]. Such conjugated polyenoic acids as α-eleostearic (9-cis,11-trans,13-trans-octadecatrienoic) acid underwent stereomutation and double bond migration when esterified with methanolic hydrogen chloride but not when reacted with boron trifluoride-methanol [185], possibly because of the shorter reaction time necessary, or when transesterified with basic reagents. On the other hand, methanol containing boron trifluoride or other strong acids was reported to cause addition of methanol to conjugated diene systems [190]. Conjugated fatty acids with hydroxyl groups adjacent to the double bond system are especially liable to rearrangement, and double bond migration, dehydration and ether formation may all occur. For example, dimorphecolic (9-hydroxy-octadeca-10-trans,12-trans-dienoic) and related acids were dehydrated to conjugated trienoic acids by strongly acidic conditions [313]. With methanol and hydrogen chloride or boron trifluoride, methoxy dienes were formed [185,286], and they were also produced on reaction with diazomethane [77]. Similarly, hydroperoxides formed during autoxidation and containing conjugated double bond systems were destroyed completely under conditions of acidic methylation and a substantial portion can be lost with base-catalysed esterification [335]. Simultaneous reduction and transesterification with sodium borohydride in methanol may help in analysis of these compounds, although the hydroperoxide group per se is lost [283,284]. α-Hydroxy acetylenic fatty acids, in contrast to their ethylenic analogues, were resistant to acid-catalysed dehydration [118].
Fatty acids with double bonds or keto groups in position 2 were reported to react with diazomethane with addition of a methylene group [31].
3. Sphingolipids and other N-acyl lipids
In sphingolipids, fatty acids are joined by an amide rather than an ester linkage to a long-chain base. The structures, occurrence and biochemistry of these compounds have been reviewed [167,352,356]. In a comprehensive analysis, it may be necessary to prepare not only the methyl esters of the component fatty acids but also the free sphingoid bases in a pure state. A method that is suitable for the first purpose may not always be suited to the second, and more than one procedure may have to be used for complete analysis of the constituents. In addition, the N-acylated lipid, N-acyl-phosphatidylethanolamine, has been found in brain and in a variety of plant tissues, and N-acyl-phosphatidylserine has been described; N-acyl amino acid derivatives have been found in several species of bacteria.
Sphingolipids and other N-acyl lipids are transesterified by vigorous acid-catalysed methanolysis, but O-methyl ethers of the long-chain bases may be formed as artefacts [46,240,347] or inversion of configuration of the functional groups at C(2) or C(3) of the bases may occur [238]. Anhydrous methanolic hydrogen chloride gave approximately 75% recovery of methyl esters from sphingomyelin after 5 hours under reflux [170], but large amounts of by-products are known to be formed from the sphingoid bases with this reagent [46,182]. In contrast, methanol containing concentrated hydrochloric acid gave much better recoveries of methyl esters and smaller amounts of artefacts from the long-chain bases [103,106,170], especially when the reagents were heated in sealed tubes in the absence of air [106,261]. All acid-catalysed transesterification procedures produce some artefacts from sphingoid bases [240], but these need not interfere with the GC analysis of methyl esters, which are easily cleaned up by TLC or column procedures in any case (see Section L) [49]. If the organic bases are not required for analysis, quantitative recovery of methyl esters can be obtained from sphingolipids by refluxing in methanol-concentrated hydrochloric acid (5:1, v/v) for five hours [170] or by heating with the reagents in a stoppered tube at 60°C for 24 hours [49]. Others have suggested the use of hydrochloric acid in aqueous butanol at 85°C for 80 minutes, followed by hydrolysis of the resultant butyl esters under mild conditions and esterification via pyrolysis of the quaternary ammonium salt [221]. Similarly, hydrolysis with 0.5M hydrochloric acid in acetonitrile-water followed by methylation has been reported to give excellent results [18]. It has been claimed that boron trifluoride-methanol reagent can transesterify amide-bound fatty acids in sphingolipids comparatively rapidly [259,260], but others [91] found that a reaction time of 15 hours at 90°C was required for complete transesterification of sphingomyelin with boron trifluoride (15%) or sulfuric acid (1 or 2.5M) in methanol. Such prolonged heating of unsaturated fatty acids in these reagents may not be advisable (see Section B.4 and Section L).
Sphingolipids are not transesterified in the presence of mild basic catalysts and this property has been utilized in the bulk preparation of sphingolipids to remove O-acyl impurities [322,349]. Basic transesterification is also of value for the quantitative release of O-acyl-bound fatty acids alone from sphingolipids and other N-acyl lipids containing both O-acyl and N-acyl fatty acids [270]. Prolonged treatment with aqueous alkali (up to 8 hours at reflux) hydrolysed sphingolipids completely (sphingomyelin must first be enzymatically dephosphorylated with phospholipase C before chemical hydrolysis), and quantitative yields of artefact-free long-chain bases were obtained [168,239,257,305]. Polyunsaturated fatty acids, which might possibly be harmed by such vigorous conditions of hydrolysis, are not normally found in significant amounts in sphingolipids so the free fatty acids released can be safely recovered and esterified by an appropriate method. Alkaline hydrolysis methods are therefore to be preferred when both the fatty acid and long-chain base components are required for analysis. Fatty acids obtained in this way can then be methylated by any of the methods described above for free acids. The hydroxyl group of 2-hydroxy acids can be methylated at the same time if reaction with methyl iodide and a base is used (see Section G.1) [63,162].
4. Sterol esters
It is well documented (but often rediscovered) that sterol esters are transesterified much more slowly than are glycerolipids. The same reagents can be employed but much longer reaction times or higher temperatures are necessary. Reaction times of an hour or more with sodium methoxide in methanol and an inert solvent at 25 to 50°C have been quoted typically [51,333,370]. While irreversible hydrolysis was found to occur under certain conditions [333], leading to a suggestion that acid-catalysed methylation should follow basic catalysis, the unwanted side effects could be avoided by the simple expedient of adding a little methyl acetate to suppress hydrolysis [51].
Acid-catalysed methylation procedures must be avoided with sterol esters (or lipid extracts containing sterols) as they react with the other constituent of the lipid, i.e. cholesterol [130,176,192,234,259,309] or phytosterols [320]. Dehydration, methoxylated and other products are formed that interfere with the analysis of polyunsaturated fatty acids by means of GC. In practice, broad peaks tended to emerge after the fatty acids of interest, often during subsequent analyses. Cholesterol itself can interfere in this way [130] and is best removed from methyl ester preparations by mini-column or TLC procedures prior to GC analysis (see Section L).
It is less well known that wax esters also are transesterified rather slowly by most reagents.
5. Selective esterification of free fatty acids in the presence of other lipids
The free or unesterified fatty acid fraction in plasma and other tissues is of special metabolic importance, so some effort has been expended to devise methods for selective esterification of this lipid class without effecting transesterification of other lipids that might be present. In general, the topic can be considered in terms of the preparation of derivatives for either GC (usually methyl esters) or HPLC (UV-absorbing or fluorescent derivatives). Methods involving selective extraction of free fatty acids or isolation by chromatography prior to esterification are not considered here.
Diazomethane [301] (see Section D.1) was one of the first methods to be used for selective methylation of free fatty acids, and it has been employed in analyses of this lipid fraction from plasma, when it has been reported to give reliable results [195,209,263,274]. It has also been utilized in a method for simultaneous extraction and methylation of free acids in plasma [274]. However, routine use has probably been discouraged in clinical laboratories because of the toxicity problems or occasional reports of side reactions. Preparation of methyl esters by reaction of free acids with N,N‘-carbonyldiimidazole and methanol also gave good results [188], but the method does not appear to have been followed by others for no clear reason. Reaction with free fatty acids from plasma and dimethylacetal-dimethylformamide (Section H.2) was judged to be unsatisfactory, because of deleterious effects on the GC column [263], and methyl iodide-potassium carbonate “proved to be less than completely reliable”. On the other hand, the latter reaction did appear to give acceptable results when a crown ether was added as catalyst [133] or with aqueous potassium hydroxide as the base [7]. Pyrolysis of the fatty acid salt of trimethyl(trifluoro-m-tolyl)ammonium hydroxide was also reported to work satisfactorily when other lipids were present [7,169]. A variation on the last procedure was used to extract and derivatize the free fatty acid fraction of vegetable oils [354].
Selective methylation of free fatty acids, after adsorption onto a strong anion exchange resin, has been effected with methanolic hydrogen chloride [62], and this method has been adapted to the analysis of free acids in milk fat [266,316]. Others have attempted to utilize the fact that free acids are esterified much more rapidly than other lipids are transesterified as a means of selective methylation of the former [206,329]. While such methods may work satisfactorily under ideal circumstances, there is no margin for error.
A quite different and novel approach involved transesterification of glycerolipids to benzyl esters with benzoyl alcohol and (m-trifluoromethyl-phenyl)trimethyl ammonium hydroxide as catalyst, with pyrolysis of the salt of the acids to the methyl esters in the injection port of the gas chromatograph [337]. Others used sodium methoxide in methanol to transesterify the ester-linked lipids, then subjected the methyl esters and the unchanged free acids to GC analysis [278].
Preparation of Esters in the Presence of Adsorbents for Thin-Layer Chromatography
It is a common practice in lipid analysis to fractionate samples by TLC so that the fatty acid composition of each lipid class can be determined. In conventional procedures, lipid classes are separately eluted from the TLC adsorbent before methylation, but it has been shown that methyl esters can be prepared in situ on the TLC adsorbent without such preliminary extraction. The elimination of this step reduces the risk of loss of sample, particularly of phospholipids which are strongly adsorbed, and of contamination of the sample by traces of impurity in the large volumes of solvent that otherwise might be necessary for elution.
Most of the common acid- or base-catalysed procedures have been adapted for this purpose. After the components have been located by spraying the developed plate with a solution of a suitable dye, such as 2′,7′-dichlorofluorescein, the methylating agent can be sprayed onto the whole plate or pipetted gently onto the individual spots. The reaction can be facilitated by warming the plate, and the esters are eventually obtained for analysis by elution from the adsorbent by a less polar solvent than might otherwise have been required to extract the original lipid component. Alternatively, the silica gel on which the lipid is adsorbed can be scraped from the TLC plate into a suitable vessel and the esterification reaction carried out in a similar manner to that when no adsorbent is present.
For example, potassium hydroxide in methanol (12%) was pipetted onto TLC plates to transesterify phospholipids [175], and this procedure was used successfully in other laboratories [295,341]. Neutral lipids have been transesterified similarly by spraying the TLC plates with 2M sodium methoxide in methanol [270,342]. Others [50,143,285,324] found that procedures involving spraying TLC plates with reagents were messy and wasteful of reagents, and that esterification could be carried out more conveniently by scraping the bands into a test tube or flask to which the esterifying reagent was added. The author once made some use of a procedure of this type but eventually found that variable amounts of water bound to the silica gel caused some hydrolysis so that the results were unreliable. This is in accord with a report that the ratio of the weight of lipid to that of silica gel must be higher than 4:1000 for consistent results [45]. In addition, there is ample evidence that cholesterol esters are esterified only slowly and unevenly in such systems [50,93]. Satisfactory results were apparently obtained when the silica gel bands were dried in vacuo over phosphorus pentoxide prior to adding sodium methoxide solution [321], but this additional step negates some of the potential advantages of this approach to methylation.
Boron trifluoride-methanol has been used to methylate unesterified fatty acids in contact with TLC adsorbents [50,136]. (Although diazomethane in diethyl ether has been sprayed onto TLC plates for the same purpose, this is much too hazardous a procedure to be recommended). Methanolic sulfuric acid [96,225], boron trifluoride-methanol [143,307], methanolic hydrogen chloride [45] and aluminium chloride-methanol [307] have been used for acid-catalysed transesterification of most lipid classes in the presence of silica gel from TLC plates. Methanol containing hydrochloric acid was found to be necessary for transesterification of sphingomyelin (see Section I.3) [50]. Again, problems were encountered mainly with cholesterol esters [50,93].
Simultaneous Extraction from Tissues and Transesterification
A common requirement of lipid analysts is to determine the total content of fatty acids and the overall fatty acid composition of a tissue of animal, plant or microbial origin. To minimize the labour involved and some potential sources of error, there have been many attempts to combine extraction of the lipids from the tissue with methylation of the fatty acid components for GC analysis. An internal standard, usually an odd-chain fatty acid, can be added to enable the total lipid content as well as the fatty acid composition to be determined; this can also compensate for any partial hydrolysis that may occur. The results have been somewhat variable, depending on the nature of the tissue, especially that of the matrix and its water content, since this has an important bearing on the yield of methyl esters. Lyophilization of the tissue has sometimes been undertaken to minimize this problem. As with other transesterification procedures, it is usually advisable to add an inert solvent such as toluene to solubilize triacylglycerols. There is a comprehensive list of references to the topic in the Literature review section of this website
Most analysts have used acidic media for simultaneous extraction and transesterification, since these are affected less by small amounts of water. For example, as long ago as 1963, it was shown that the fatty acids of bacteria could be extracted efficiently and methylated with boron trichloride in methanol [1], a finding confirmed later [85]. A distinctive low temperature method, utilizing methanol-sulfuric acid in diethyl ether, was used to methylate lipids in animal tissues [86]. Cereal grains of various kinds were ground to a powder and the lipids transesterified directly with 10% boron trichloride [27] or 1 to 2% sulfuric acid in methanol [69,348]. 5% Methanolic hydrogen chloride was used similarly in experiments with ruminant feeds, digesta and faeces [273], and with added toluene for various food materials [334]. Rat serum and brain tissues were first dehydrated by reaction with dimethoxypropane; excess solvents were removed by evaporation (so artefact formation (see Section B.2) was not troublesome), before the tissue was extracted and methylated with methanolic hydrogen chloride [192]. Alternatively, lipoprotein fractions from plasma were lyophilized prior to methylation with boron trifluoride-methanol [299]. On the other hand, Lepage and Roy [204,205] have suggested that such a step is not necessary. Provided that the water content of the medium was not allowed to rise above 5%, much better recoveries of fatty acids were obtained by extraction/transesterification with methanol-hydrogen chloride-benzene than with standard extraction procedures. Plasma, milk, adipose tissue, bile, liver and other tissues were analysed, and the method has been adapted for the specific methylation of the free fatty acid fraction of plasma [206]. The validity of this procedure was confirmed by others and it was applied to further types of sample [319,350]. A related procedure has been used with green leaf tissue [42].
In an interesting alternative approach, isopropanol or hexane-isopropanol mixtures were used to extract the lipids from such materials as cheese, other dairy products and erythrocytes [280,359,360], anhydrous sodium sulfate was used to remove the water and then concentrated sulfuric acid was added directly to the solution as a catalyst to effect transesterification with formation of isopropyl esters for GC analysis.
Base-catalysed methods have been little used for this specific purpose, but satisfactory results were obtained with small samples of materials with a low water content, such as crushed rapeseed [141], soy meal, cheese and buttermilk blends by means of direct methylation with sodium methoxide [213]. Similarly, trimethylphenylammonium hydroxide has been utilised to transesterify plasma extracts for the determination of phytanic acid [61]. Whole bacterial cells have been subjected to methylation or pyrolytic methylation with some success, i.e. by reaction with tetramethylammonium hydroxide [89,246] or trimethylsulfonium hydroxide [264,265].
Free fatty acids in plasma have been transesterified directly and selectively by methylation with diazomethane in methanol [274].
As an alternative, many analysts have used either acidic or basic treatments to improve the extractabilty of fatty acids from tissue matrices before proceeding to methylation, but this is out with the scope of the present review.
Artefacts of Esterification Procedures
Vigilance must be exercised continuously to detect contamination of samples by impurities in the reagents or from any other extraneous source. Middleditch [241] has compiled an exhaustive treatise on compounds that can be troublesome in chromatographic analyses (including mass spectral information). In addition, the samples themselves may be a source of contaminants or artefacts, for example from the fatty acids per se (see Section I.2), endogenous cholesterol, other sterols or their esters (see Section I.4), and other lipids. Thus, dimethyl acetals are formed from plasmalogens by the reaction of acidic transesterifying reagents, and these tend to elute just ahead of the corresponding esters (generally C16 and C18) on GC. All solvents (including water) and reagents should be of the highest grade and may have to be distilled before use to remove nonvolatile impurities, especially when preparing very small quantities of esters. Extraneous substances can be introduced into samples from a variety of sources, for example filter papers, soaps, hair preparations, tobacco smoke and laboratory grease, and care must be taken to recognize and avoid such contaminants [15,210,292].
Phthalate esters, used as plasticisers, are probably the most common contaminants and are encountered whenever plastic ware of any kind is in contact with solvents, lipid samples, reagents and even distilled water [32,66,153,210,275,276,279]. They can enter preparations via the brief contact between disposable pipette tips and reagents. In addition, phthalate esters can interact with transesterification reagents to give mono- and sometimes dimethyl esters, basic catalysts reacting more rapidly than acids [308]. In GC analysis, the precise point of elution relative to fatty acid derivatives is dependent on the nature of the phthalate ester and the stationary phase, but typically is in the same range as the C18 to C22 fatty acids. In HPLC, the precise elution point is dependent on the mode of chromatography, and the problem is especially troublesome with UV detection systems.
Samples containing polyunsaturated fatty acids should be handled under nitrogen whenever possible, and antioxidants such as 2,6-di-tert-butyl-4-methylphenol (BHT) may be added to solvents and reagents to minimize autoxidation [363]. In GC analyses, BHT emerges as a sharp peak, which can interfere with the analysis of methyl myristate with packed columns but not usually when fused silica capillaries are used; in HPLC, it often emerges close to the solvent front where it can be a nuisance with UV detection. Boron trifluoride is known to interact with BHT to produce methoxy derivatives as artefacts that co-elute with methyl pentadecanoate or hexadecenoate on GC analysis [33,80,131,255]. This does not appear to be a problem with other acidic reagents.
Ethyl esters were found in methyl ester preparations, when chloroform containing ethanol as a stabilizer was employed as a solvent to facilitate transesterification [159]. During GC analysis per se, artefact peaks have been produced when traces of basic transesterification reagents, remaining in samples, were introduced into GC columns [326].
Non-lipid contaminants or non-ester by-products of esterification reactions, for example cholesterol, can be removed by preparative TLC on plates coated with silica gel G and developed in a solvent system of hexane-diethyl ether (9:1, v/v), though there may be some selective loss of short-chain esters if these are present. BHT migrates with the solvent front and is closely followed by the required esters. Alternatively, a short column of silicic acid or Florisil™, in a disposable Pasteur pipette say, can be used and the esters recovered by elution with hexane-diethyl ether (95:5, v/v). Alumina column chromatography has been recommended for the same purpose [234]. Dimethyl acetals formed from plasmalogens have been separated from methyl esters by preparative TLC with toluene [224] or dichloroethane [355] as mobile phase, when esters migrate more rapidly.
The Choice of Reagents – a Summary
Methyl esters are by far the favourite derivatives for GC analysis of fatty acids, although a case can be made for isopropyl or butyl esters in some circumstances. One of the first considerations when deciding which procedure to adopt for preparing ester derivatives for GC is the lipid composition of the samples to be analysed. If these are free fatty acids alone, mixtures containing significant amounts of free acids or mixtures of unknown composition that might contain free acids, then acid-catalysed procedures are generally preferred. The newer quaternary ammonium reagents, which can be used both for esterification and transesterification, appear to be promising alternatives. On the other hand, alkaline transesterification procedures are so rapid and convenient that they must be considered for mixed lipid samples that contain little or no unesterified fatty acids or for single lipid classes containing ester-bound fatty acids. If a single method is required for use with all routine lipid samples, hydrogen chloride (5%) or sulfuric acid (2%) in methanol, despite the comparatively long reaction times needed, is probably the best general-purpose reagent available. The author, does not find it inconvenient to have more than one method in routine use in his own laboratory, i.e. sulfuric acid in methanol for those samples containing free fatty acids, and sodium methoxide in methanol for all other lipid classes with the exception of sphingolipids (for which special procedures are in any case necessary (see Section I.3)).
A further consideration when deciding on a reagent is the fatty acid composition of the samples to be esterified. If short-chain fatty acids (Section I.1) or others of unusual structure (Section I.2) are present, appropriate methods must be adopted. Polyunsaturated fatty acids must always be handled with care and should not be subjected to more vigorous conditions than are necessary. It should be recognized that reagents that are perfectly satisfactory when used under optimized conditions can be destructive to fatty acids if used carelessly.
Similar restrictions apply in the preparation of derivatives for HPLC. Here there is no consensus as to which is the best derivative from a chromatographic standpoint, and the choice may be determined to some extent by the nature of the detector available to the analyst.
Acknowledgements: This review was published as part of a programme funded by the Scottish Executive Environment and Rural Affairs Department.
Abbreviations: BHT, 2,6-di-tert-butyl-4-methylphenol; GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; TLC, thin-layer chromatography.
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…