Selected Topics in the Analysis of Lipids
Methods for the structural characterization of fatty acid methyl esters (FAME) by mass spectrometry (MS) are restricted to a few techniques that are rare in modern MS laboratories. The utility of many of these methods for identifying double bond position is limited. Conventional electron impact (EI) MS with 70 eV electrons yields peaks that are mostly not specific to structure because of diffuse charge localization that drives rearrangements and fragmentation. A solution to the charge localization problem is the conversion of FAME to dimethyloxazoline (DMOX) or picolinyl esters, discussed elsewhere in the Lipid Library. The strong charge-localizing properties of the derivative group yield many low-abundance ions that are indicative of single and double bonds. Each derivative requires its own chromatography conditions and in addition the lower molecular weight esters have lower volatility than FAME. The necessities of different derivative chemistry and chromatography are inconvenient for most labs, and the differing retention times of FAME versus the corresponding DMOX or picolinyl derivative can make matching minor components impossible. Moreover, the large number of low-abundance fragments that are not characteristic of a particular fatty acid makes selected ion monitoring nearly impossible.
Covalent adduct chemical ionization (CACI) MS was developed as a convenient and rapid method for unequivocal identification of FAME double bond structure without the need for prior chemical rederivatization. An acetonitrile-derived reagent ion interacts with the double bonds of FAME and subsequent fragmentation yields a small number of predictable peaks that are indicative of double bond location for most FAME. The well established patterns of FAME fragmentation in CACI MS and tandem MS enable an interpretation and, in most cases, allow structures to be determined without the need for comparison against known standards.
Key practical advantages of CACI MS/MS for FAME analysis over rederivatization methods include:
- FAME are analyzed directly; no additional chemical treatment of FAME mixtures is required.
- GC conditions (column, temperature program and sample concentration) optimized for FAME analysis are used, thereby obviating the need to use separate conditions for other fatty acid esters.
- Definitive identification of FAME chemical structure is obtained based on interpretable fragmentation patterns indicative of hydrocarbon chain length, degree of unsaturation and double bond position.
CACI MS and tandem MS analysis of FAME delivers particularly facile information of interest in FAME analyses:
- Conclusive detection and identification of FAME, compared with other lipids (e.g. squalene, ethyl esters) based on pattern of ions in MS-1 spectra.
- Identification of FAME as being saturated, monoenoic or polyenoic.
- Ruling out peaks as being monoenoic, dienoic or polyenoic FAME.
- FAME can be assigned easily compared, for instance, with a GC-FID analysis, because there is no change in retention time as there is with rederivatization methods; this is of particular importance for FAME of low relative concentration.
- Identification of double bond position for all homoallylic FAME.
- Identification and quantification of individual cis and trans monoenoic FAME.
- Comprehensive identification of double bond position and geometry for conjugated linoleic acid FAME.
CACI MS-1 of FAME
All of our studies have been performed on a Varian gas chromatograph coupled to a Saturn 3 or Saturn 2000 mass spectrometer with a 3D internal ionization trap (Varian Inc., Walnut Creek, CA). A benefit of the 3D internal ionization trap is that it maintains relatively high concentrations of ionized reagent gas in the trapping region, compared with external ionization CI sources as are required of quadrupole MS and in external ionization traps.
Acetonitrile derived reagent ion and mechanism of [M+54]+ ion formation
Acetonitrile (CH3CN, m/z 41) maintained under CI conditions yields the expected dominant ions, m/z 40 (CH2CN+) and m/z 42 (CH4CN+), as well as an additional ion at m/z 54, appearing as a decomposition product from the reaction of acetonitrile with an ion of itself. The m/z 54 ion is formed from the net transfer of a CH3 and yields (1 methyleneimino) 1 ethenylium (MIE), with the structure CH2=C=N+=CH2. This ion reacts rapidly with double bonds of FAME and produces an ion 54 Da above the mass of the parent FAME, referred to as the [M+54]+ ion. Mechanistic studies have determined that MIE forms a four-membered heterocyclic ring with monoenes and homoallylic polyenes, and a six-membered ring structure with conjugated dienes (Van Pelt et al., 1999).
MS-1 mass spectra
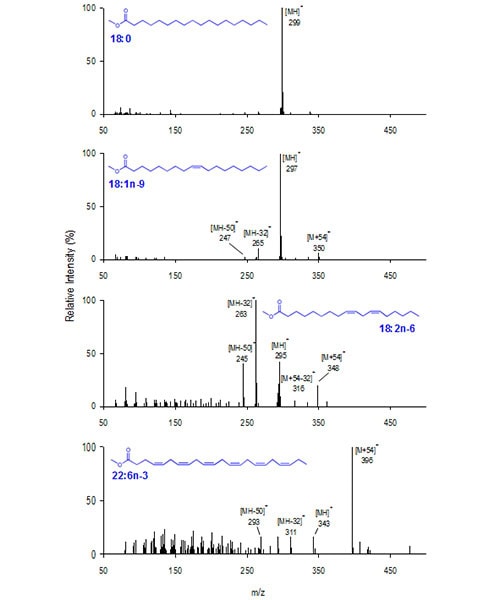
Figure 1 presents MS-1 spectra under CACI conditions for a representative saturate, monoene, diene, and polyene: methyl stearate (Me18:0), oleate (Me18:1n-9), linoleate (Me18:2n-6), and docosahexaenoate (Me22:6n-3). The Me18:0 MS-1 spectrum has only a protonated molecular ion, [MH]+ at m/z 299. The MS-1 spectrum for Me18:1 is similar to that of all unsaturated FAME. Four characteristic ions are found corresponding to the protonated molecular ion ([MH]+), the [MH]+ ion with a loss of 32 Da (- CH3OH) or 50 Da (- CH3OH, – H2O) and the [M+54]+ ion. For monoenes, the MS-1 spectrum is dominated by MH+, while the [M+54]+, [MH−32]+ and [MH−50]+ ions are found at lower intensities. Similarly, MS-1 for Me18:2n-6 shows the same four ions and with greater relative intensity for [M+54]+ and [MH−50]+ compared with the corresponding ions from monoene FAME. Finally, the MS-1 of Me22:6n-3 shows dominant [M+54]+ ion at m/z 396 relative to the [MH]+ ion. For highly unsaturated FAME, the [M+54]+ ion is often the most prominent ion in the mass spectrum consistent with the enhanced probability that MIE reacts with the greater number of double bonds per molecule.
Figure 1. Stearate (Me18:0), oleate (Me18:1n-9), linoleate (Me18:2n-6) and docosahexaenoate (Me22:6n-3) CACI MS1 mass spectra.
Homoallylic versus conjugated double bond structure
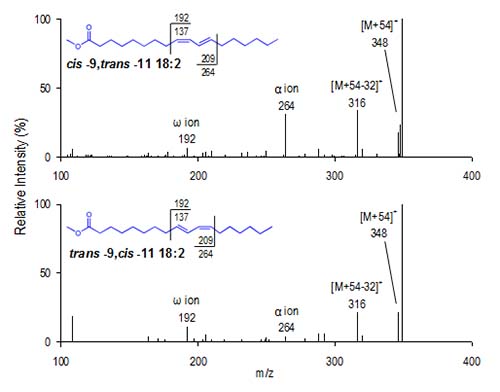
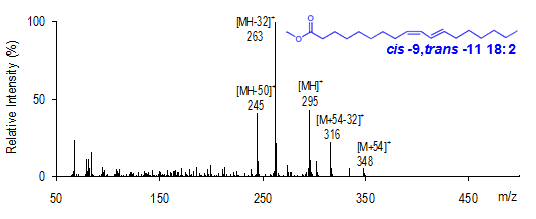
MS-1 ion intensities reveal details of double bond structure. The relative intensity of the [M+54]+ ion, compared with a fifth ion corresponding to [M+54-32]+ (- CH3OH), provides structural information concerning the presence and arrangement of a conjugated double bond system versus the more common homoallylic (methylene-interrupted) chain. For homoallylic FAME, the ratio of [M+54]+/[M+54−32]+ is greater than 1.0, whereas the mass spectra of conjugated dienoic FAME yield [M+54]+/[M+54-32]+ less than 1.0. For example, the mass spectrum for cis-9,trans-11-18:2 is illustrated in Figure 2. The ratio of [M+54]+/[M+54−32]+ for this conjugated FAME is 0.2. Conversely, the [M+54]+/[M+54−32]+ ratio for linoleate in Figure 1 is about 4.0.
Figure 2. cis-9,trans-11-18:2 CACI MS-1 mass spectrum.
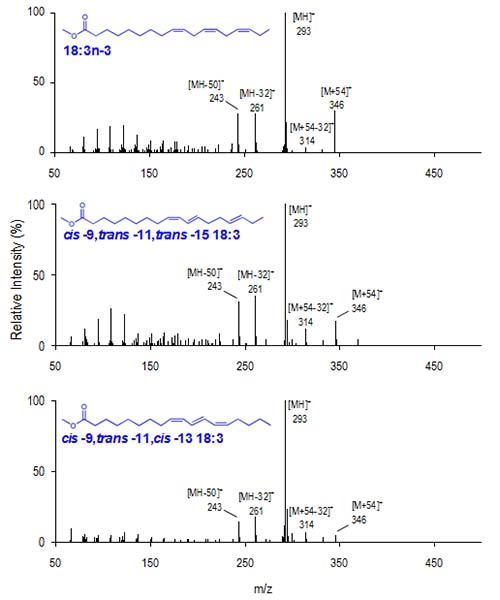
Figure 3 presents mass spectra for the homoallylic 18:3n-3, cis-9,trans-11,trans-15-18:3, a partially conjugated FAME identified in ruminant milk and cis-9,trans-11,cis-13-18:3, punicic acid, a fully conjugated FAME originating from pomegranate seed oil. The [M+54]+/[M+54−32]+ ratio for these FAME are 8.4, 1.4 and 0.8, respectively, indicative of homoallylic, partially conjugated and fully conjugated double bond systems (Lawrence and Brenna, 2005).
Figure 3. CACI MS-1 for three C18 triene FAME. Top: 18:3n-3, Center: cis-9,trans-11,trans-15-18:3, Lower: cis-9,trans-11,cis-13-18:3. The [M+54]+/[M+54−32]+ ratio for these isomers is 8.4, 1.4 and 0.8, respectively, indicative of homoallylic, partially conjugated and fully conjugated FAME.
CACI MS/MS (MS-2) of [M+54]+
The position and in special cases, geometry, of double bonds is found in the collisional dissociation products of the [M+54]+ ion, formed during the second stage of MS (MS/MS or MS-2). The [M+54]+ ion yields two product ions, of high abundance depending on collision conditions, that are characteristic of double bond structure in all homoallylic FAME. They carry the carboxyl or terminal methyl carbons and are referred to as the α and ω ions, respectively.
The principal is illustrated in Figure 4 for two isomeric monoenes, Me-trans-11-18:1 and Me18:1n-9. MS-1 spectra of the two monoenes produce identical spectra with the four ions characteristic of FAME. MS-2 of the isolated [M+54]+ ion yields different ions that are diagnostic of structure: The trans-11 isomer yields m/z 280 and 180 for α and ω ions, respectively, while the n-9 isomer yields m/z 252 and 208.
Figure 4. MS-1 and MS-2 of [M+54]+ for two monoene isomers, trans-11-18:1 and Me18:1n-9. MS-1 spectra are indistinguishable, despite the different double bond geometry. Diagnostic ions specific to each isomer are found in the MS-2 spectra. Numbers inside the horizontal line fragment designations reflect the mass of the parent ion fragment, while the numbers outside the lines are the actual fragment masses considering addition of 54 Da, plus or minus 1 for a H transfer.
MS-2 fragments for homoallylic double bond systems
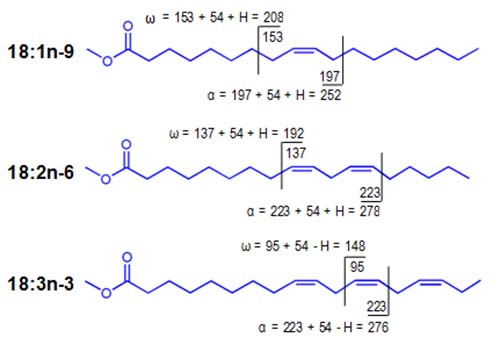
Heuristic rules for the collisional dissociation of homoallylic FAME are illustrated in Figure 5, and have been tested with FAME >having as many as eight double bonds (e.g. 28:8n-3) (Van Pelt and Brenna, 1999). Fragment ions of [M+54]+ for homoallylic double bond systems are specific to the degree of unsaturation in the parent FAME. For monoenes, fragmentation occurs allylic, or just beyond the methylene group, to the double bond and an H• (atom) is transferred to the ion. Fragmentation of dienes occurs at positions vinylic (i.e. adjacent) to the double bonds and again an H• is transferred to the ion. For dienes and higher polyenes, fragmentation occurs internal to the double bond system, at sites allylic to the first double bond from both the carboxyl and methyl ends of the parent FAME, and an H• is transferred away from the ion.
Figure 5. Rules for the formation of α and ω diagnostic ions from homoallylic FAME in CACI MS/MS
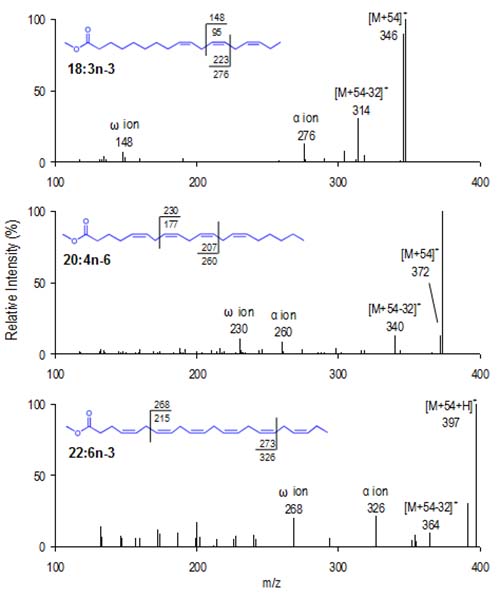
Figure 6 shows CACI MS-2 spectra for a triene, tetraene, and hexaene to illustrate the commonality in polyene spectra. The α and ω diagnostic ions result from predictable fragmentation, and data for the ions corresponding to specific homoallylic FAME structures appear in tabulated form here in .pdf format.
Figure 6. CACI MS-2 spectra of three homoallylic FAME, 18:3n-3 (top panel), 20:4n-6 (middle panel), 22:6n-3 (bottom panel). MS-2 spectra yield four characteristic ions, the α and ω ions, as well as the [M+54]+ ion and [M+54−32]+. A protonated [M+54]+ ion is commonly detected with higher levels excitation energy, as shown for 22:6n-3.
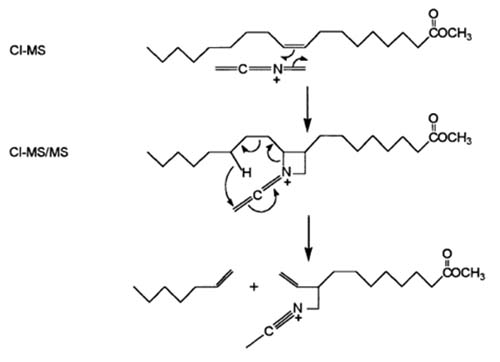
Chemical structures for the reaction of a FAME with MIE, with subsequent dissociation to diagnostic ions, is presented in Figure 7 for a monoene. The [M+54]+ ion has a heterocyclic four-membered ring that includes the N of MIE. Dissociation to form the α-diagnostic ion is shown. Reaction of MIE in an antiparallel sense followed by analogous dissociation yields the ω-diagnostic ion.
Figure 7. Reaction of MIE with a monoene, with subsequent dissociation to form the α-diagnostic ion.
Figure 8 illustrates the systematic shift in mass of diagnostic ions in MS-2 for structurally related FAME. Starting from the methyl ester, Me16:2n-4 is structurally identical to Me18:2n-6 except for the absence of –CH2–CH2– (28 u) at the ω methyl end. The α-diagnostic ions for the two FAME are identical at m/z 278; the lower molecular weight Me16:2n-4 has an ω-diagnostic ion at m/z 164 while this ion appears at m/z 192 (+28 u) for Me18:2n-6. Similarly, Me18:2n-6 and Me20:2n-6 are structurally identical except for –CH2–CH2– at the carboxyl end. Here, the ω-diagnostic ions are at the same m/z 192, while the individual FAME can be distinguished by their α-diagnostic ions, m/z 278 versus m/z 306.
Figure 8. Mass spectra of three dienes differing by hydrocarbon chain length.
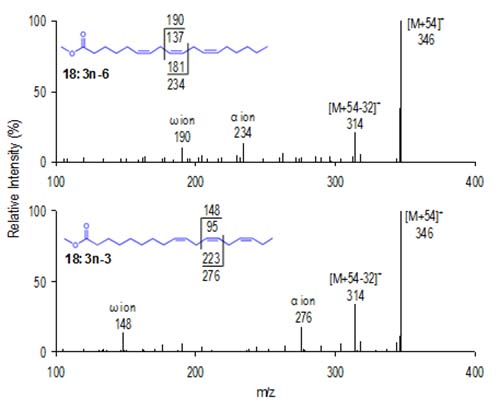
Figure 9 presents MS-2 spectra for γ-18:3 and α-18:3. As the homoallylic triene double bonds shift between the ω3 and ω6 positions, both diagnostic ions shift and enable facile identification of the two isomers.
Figure 9. CACI MS-2 spectra of two 18:3 isomers.
Facile selected/reconstructed ion monitoring: trans monoene FAME quantitative analysis
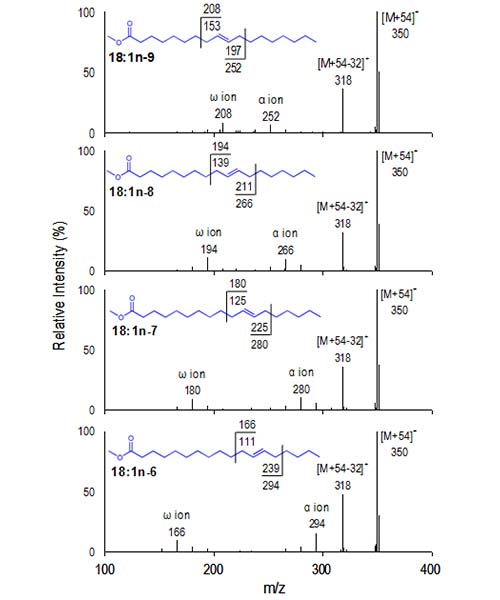
Quantification of individual trans fatty acids by conventional gas chromatography is complicated by the incomplete separation of coeluting isomers, thus requiring long temperature programs. CACI-MS/MS monitoring of diagnostic ions provides unique advantages for separations and quantitative analysis of FAME. Here, diagnostic ions can be used to quantify individual 18-carbon cis and trans isomers in MS/MS with a short temperature program (Brenna, 2005), and the [M+54]+ ion for 18:1 is isolated and collisionally dissociated. Figure 10 presents MS-2 spectra for four monoene FAME and shows that they yield unique diagnostic ions suitable for plotting and quantification.
Figure 10. CACI MS-2 spectra of four selected 18:1 isomers. Diagnostic ions are unique for individual isomers and thus can be summed and plotted to reconstruct a structure-specific chromatogram.
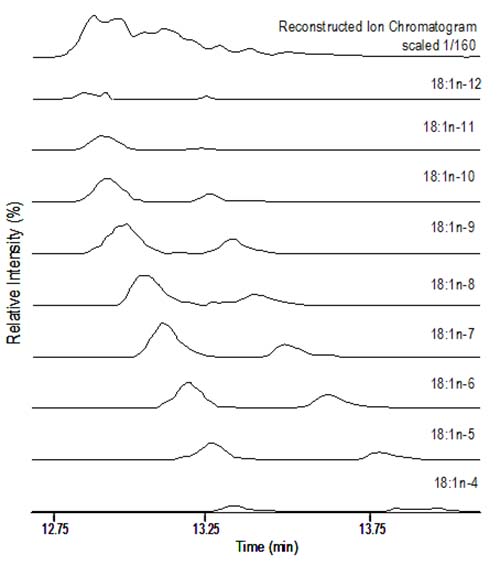
Figure 11 presents plots of reconstructed ion chromatograms based on diagnostic ions for various monoene isomers found in partially hydrogenated vegetable oil. For this sample, two peaks are found for all isomers, with the trans peak appearing at lesser retention time than the cis isomer. Because each isomer contains one double bond, detection sensitivity is similar among isomers. Peak areas for the diagnostic ions of individual FAME isomers are plotted, quantified and taken as a proportion of the total peak area.
Figure 11. Region of chromatogram for partially hydrogenated vegetable oil FAME following CACI MS/MS. The top panel represents the reconstructed ion chromatogram for total 18:1 FAME. Lower panels plot the diagnostic ions for individual 18:1 FAME, with trans 18:1 isomers eluting before the respective cis isomers. Peak areas for individual isomers are integrated and quantified as a proportion of the total peak area.
Conjugated linoleic acid (CLA) FAME: Double bond position and geometry (cis/trans)
About 30 CLA isomers have been identified in the milk and meat of ruminant animals, with cis-9,trans-11-18:2 and trans-7,cis-9-18:2 being the most abundant. Identification and resolution of CLA by conventional gas chromatography has proven to be a great challenge due to the wide range of isomers and small structural differences among them.
CLA are particularly amenable to analysis by CACI-MS/MS (Michaud et al., 2003). Similar to homoallylic dienoic FAME, fragmentation of CLA in MS-2 occurs allylic to the double bonds, and the mass spectra yield α and ω diagnostic ions indicative of double bond position. Surprisingly, the ratio of the α and ω ions (i.e. α/ω ratio) reveals the geometry of the double bonds, with the fragment arising from cleavage adjacent to the trans bond more prominent than the fragment adjacent to the cis bond. Thus, cis-trans isomers give an α/ω ratio ≥ 4 while trans-cis isomers yield a ratio less than 1.0. MS-2 mass spectra for cis-9,trans-11-18:2 and trans-9,cis-11-18:2, with α/ω ratios of 5 and 0.4, respectively, are presented in Figure 12. CLA isomers of common geometry, cis/cis and trans/trans isomers, yield similar α/ω ratios and cannot be distinguished by MS-2 alone. Informal observations are that they can be distinguished by the MS-1 [M+54]+/MH+ intensity ratios, where the trans-trans isomer is formed at greater relative abundance than the cis-cis isomer (Lawrence and Brenna, 2005).
Figure 12. CACI MS/MS mass spectra of cis-9,trans-11-18:2 (top panel) and trans-9,cis-11-18:2 (bottom panel). The ratio of α/ω diagnostic ions for these isomers is 5.0 and 0.4, respectively, indicative of cis,trans and trans,cis double bond geometry.
Non-methylene interrupted (NMI) and conjugated linolenic acid FAME
CACI positional assignment in polyene NMI-FAME spectra is more complex, in part because numerous [M+54]+ isomers with coupled dissociation pathways may be present. Conjugated polyenes, FAME with double bond systems isolated by more than one –CH2–, and triple-bond containing FAME all give unique fragmentation, with a few exceptions (Lawrence and Brenna, 2005, Gómez-Cortés et al., 2009). Conclusive structural evidence for a unique structure for these FAME is best accomplished with standards.
Importantly, however, the absence of MS-1 and MS-2 fragments indicative of homoallylic or CLA FAME rules out these common structures. Comparison with existing reference spectra and application of the fragmentation rules developed for simple double bond systems enables identification by interpretation.
References
- Brenna, J.T. Double bond localization in fatty acid methyl esters by covalent adduct chemical ionization (CACI) tandem mass spectrometry. Lipid Technology, 17, 231-234 (2005).
- Brenna, J.T. Structural analysis of unsaturated fatty acid methyl ester isomers with acetonitrile covalent adduct chemical ionization (CACI) tandem mass spectrometry. In: Lipids Analysis and Lipidomics: New Techniques and Applications. pp. 157-172 (eds. M.M. Mossoba, J.K.G. Kramer, J.T. Brenna and R.E. McDonald, AOCS Press, Champaign, IL) (2006).
- Gómez-Cortés, P., Tyburczy, C., Brenna, J.T., Juárez, M. and de la Fuente, M.A. Characterization of cis-9 trans-11 trans-15 C18:3 in milk fat by GC and covalent adduct chemical ionization tandem MS. J. Lipid Res., 50, 2412-2420 (2009) (DOI: 10.1194/jlr.M800662-JLR20).
- Lawrence, P.A. and Brenna, J.T. Acetonitrile covalent adduct chemical ionization (CACI) mass spectrometry for double bond localization in non-methylene-interrupted polyene fatty acid methyl esters. Anal. Chem., 78, 1312-1317 (2006) (DOI: 10.1021/ac0516584).
- Michaud, A.L., Yurawecz, M.P., Pierluigi, D., Corl, B.A., Bauman, D.E. and Brenna, J.T. Identification and characterization of conjugated fatty acid methyl esters of mixed double bond geometry by acetonitrile chemical ionization tandem mass spectrometry. Anal. Chem., 75, 4925-4930 (2003) (DOI: 10.1021/ac034221+).
- Van Pelt, C.K. and Brenna, J.T. Acetonitrile chemical ionization tandem mass spectrometry to locate double bonds in polyunsaturated fatty acid methyl esters. Anal. Chem., 71, 1981-1989 (1999) (DOI: 10.1021/ac981387f).
- Van Pelt, C.K., Carpenter, B.K. and Brenna, J.T. Studies of structure and mechanism in acetonitrile chemical ionization tandem mass spectrometry of polyunsaturated fatty acid methyl esters. J. Am. Soc. Mass Spectrom., 10, 1253-1262 (1999).
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…
![MS-1 and MS-2 of [M+54]+ for two monoene isomers, trans-11-18:1 and Me18:1n-9](https://www.aocs.org/wp-content/uploads/2019/07/Fig4_ms1_ms2.jpg)