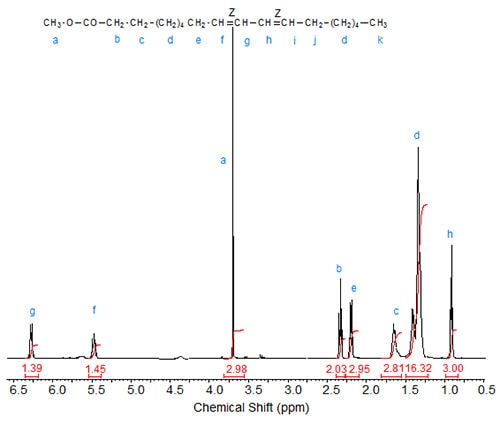
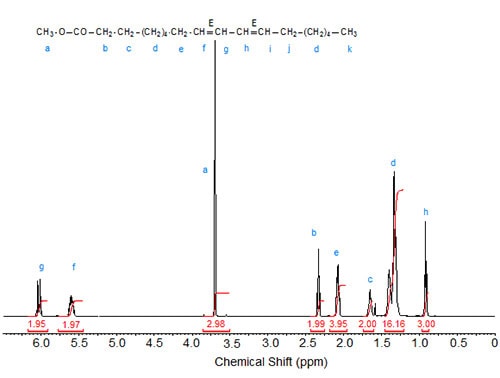
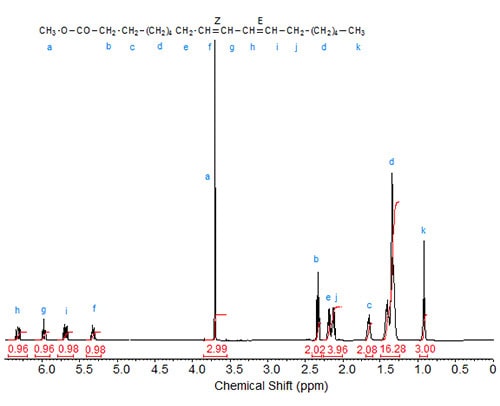
Figures 1 to 3 depict the 1H-NMR spectra of three conjugated linoleic acid methyl esters, namely three isomers of methyl 9,11-octadecadienoate [cis,cis– (9Z,11Z); trans,trans– (9E,11E); cis,trans– (9Z,11E), respectively] that can serve as models for other CLA esters.
Figure 1. 1H-NMR spectrum of methyl 9(Z),11(Z)-octadecadienoate.
The characteristic shifts are relative to deuterochloroform (CDCl3) at 7.295 ppm and are given in Table 1.
| Table 1. Assignments of characteristic signals in the spectra of 9,11-octadecadienoic acid isomers (see Figs. 1 to 3): | |||||
| Allylic protons (C8, C13) |
C9 | C10 | C11 | C12 | |
|---|---|---|---|---|---|
| 9Z,11Z | 2.08 | 5.47 | 6.27 | 6.27 | 5.47 |
| 9E,11E | 2.20 | 5.59 | 6.03 | 6.03 | 5.59 |
| 9Z,11E | 2.18 (cis), 2.13 (trans) | 5.32 | 5.97 | 6.34 | 5.69 |
Figure 2. 1H-NMR spectrum of methyl 9(E),11(E)-octadecadienoate.
Figure 3. 1H-NMR spectrum methyl 9(Z),11(E)-octadecadienoate.
The “inner” olefinic protons at C10 and C11 are shifted downfield compared to the “outer” olefinic protons at C9 and C12. Also, in accordance with the above discussion on oleic acid vs. elaidic acid, the trans olefinic protons are downfield from the cis protons in the two sets, external and internal protons. Similarly, the signals of the allylic protons of the cis isomers are downfield from the trans isomers, especially visible in the spectrum of the mixed Z,E isomer. In the case of identical double bond configuration, the signals of the two internal olefinic protons overlap as do the signals of the two external olefinic protons.
The 1H-NMR analysis of the compounds depicted here was also described in the literature (Lie Ken Jie et al., 1997; Lie Ken Jie, 2001). The corresponding data (also CDCl3, 300 MHz) are:
- Four peaks for the 9Z,11E isomer, namely, two multiplets (5.32, 5.65) and two triplets (5.82, 6.24) attributable to the outer (9, 12) and inner (10, 11) protons, respectively.
- For the 9Z,11Z isomer, a multiplet at 5.40 ppm assigned to the outer positions 9, 12 and a double of doublet 6.22 caused by the inner positions 10, 11.
- In the 9E,11E isomer, the outer protons at 5.56 ppm and inner protons at 5.96 ppm.
Literature:
- Lie Ken Jie, M.S.F., Pasha, M.K. and Alam, M.S. Synthesis and nuclear magnetic resonance properties of all geometric isomers of conjugated linoleic acids. Lipids, 32, 1041-1044 (1997).
- Lie Ken Jie, M.S.F. Analysis of conjugated linoleic acid esters by nuclear magnetic resonance spectroscopy. Eur. J. Lipid Sci. Technol., 103, 628-632 (2001).
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…