Figures 1-3 depict the spectra of an ethyl, propyl and butyl ester, respectively. The spectra of these esters permit comparisons of the changes in the spectra of the alkyl group with increasing numbers of CH2 groups.
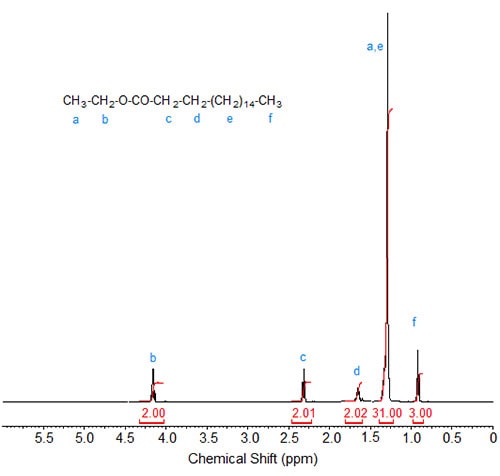
Ethyl esters. In the spectrum of ethyl stearate (Fig. 1), the singlet of the methyl ester protons is replaced by a quartet at about 4.2 ppm caused by the methylene protons of the ethyl group. The signal of the methyl protons in the ethyl group is obscured by the large CH2 signal, giving it an integration value of 31 (14 CH2 from the stearic acid chain and three methyl protons from the ethyl group).
Figure 1. 1H-NMR spectrum of ethyl stearate.
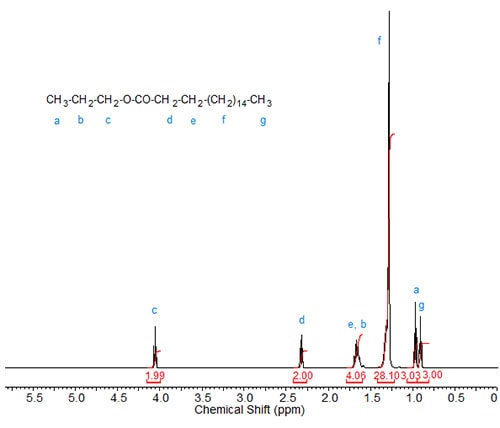
Propyl esters. With propyl stearate (Fig. 2), the methylene group attached to the oxygen in the ester group is now a triplet at approximately 4.08 ppm. The signal of C3 in the fatty acid chain is now overlapped by the second methylene group in the propyl ester, giving it a theoretical integration value of 4. The methyl group in the propyl ester is more remote from the oxygen, shifting it upfield close to the signal of the terminal methyl of the fatty acid chain. The theoretical integration value of the large methylene signal is therefore reduced to 28.
Figure 2. 1H-NMR spectrum of propyl stearate.
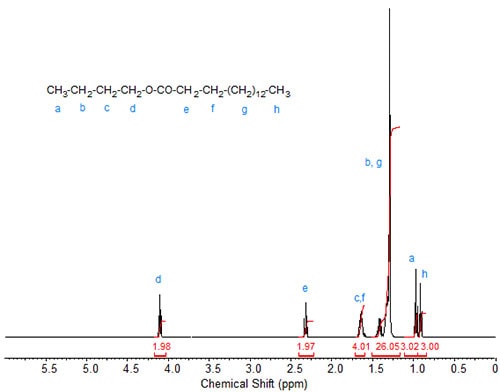
Butyl esters. With butyl palmitate (Fig. 3), an additional signal caused by the terminal methylene in the butyl group is visible and is integrated in the figure together with the methylenes in the fatty acid chain (theoretical integration value 26; 12 CH2 in the fatty acid chain plus two butyl methylene protons). Otherwise, the various alkyl esters have virtually no effect on the signals caused by the protons in the fatty acid chain.
Figure 3. 1H-NMR spectrum of butyl palmitate.
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…