Chemical shifts (ppm) for several carbon atoms in oleic acid and some of its derivatives are summarised in the Table below. There are diagnostic signals for these classes of long-chain compounds.
| Carbon atom | a | b | c | d | e | f |
|---|---|---|---|---|---|---|
| Acid | 180.43 | 34.13 | 24.70 | – | – | – |
| Methyl ester | 174.28 | 34.10 | 25.01 | 51.39 | – | – |
| Glycerol ester (α) | 173.23 | 34.07 | 34.91 | – | – | – |
| Glycerol ester (β) | 172.82 | 34.23 | 24.93 | – | – | – |
| Alcohol | – | – | – | 63.03 | 32.81 | 25.76 |
| Acetate | 171.16 | 20.99 | – | 64.63 | 28.66 | 25.96 |
| Wax ester | 173.97 | 34.43 | 25.06 | 64.38 | 28.69 | 25.97 |
| Amide (i) | 76.37 | 36.02 | 25.57 | – | – | – |
| Nitrile (ii) | 119.77 | 25.41 | 17.12* | – | – | – |
| * Also 28.68 (C4) 28.77 (C5) (i) Huang et al. (1997) have given different values for the amide of 12-hydroxystearic acid: 176 (C1) and 33.1 (C2). (ii) Information on more complex nitriles is given in many papers devoted to the synthesis of polyunsaturated fatty acids since they are important intermediates. |
||||||
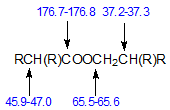
Characteristic chemical shifts have also been reported for di-Guerbet esters. These are branched-chain compounds made from Guerbet acids and Guerbet alcohols and have the structure shown below where the four R groups (C4 – C12) may differ from each other.
References
- Gunstone, F.D . High resolution 13C-NMR spectra of long-chain acids, methyl esters, glycerol esters, wax esters, nitriles, amides, alcohols, and acetates. Chem. Phys. Lipids, 66, 189-193 (1993).
- Huang, K.-K., Keudell, K.C., Klopfenstein, W.E., Wen, L., Bagby, M.O., Vesonder, R.F., Norton, R.A. and Weisleder, D. Biotransformation of 12-hydroxyoctadecanoic acid to 12-hydroxyoctadecanamide by Bacillus cereus 50. J. Am. Oil Chem. Soc., 74, 601-603 (1997).
- Knothe, G. and Carlson, K.D. Synthesis, mass spectrometry, and nuclear magnetic resonance characterization of di-Guerbet esters. J. Am. Oil Chem. Soc., 75, 1861-1866 (1998).
- Vieville, C., Mouloungui, Z. and Gaset, A. Synthesis and analysis of C1-C18 alkyl oleates. Chem. Phys. Lipids, 75, 101-108 (1995).
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…