The preservative wars
By Laura Cassiday
February 2013
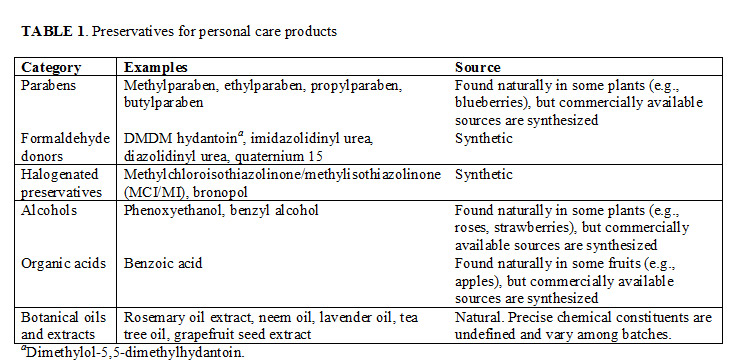
• Preservatives are necessary to curb microbial growth in lotions, creams, makeup, and other personal care products.
• Concerns about traditional preservatives have created a substantial market for “natural” preservatives derived from plant sources, but there are many definitions of “natural.” Whether natural preservatives are safer than their traditional counterparts is still under debate.
• The safety of any preservative depends on the dose, and “natural” preservatives can present challenges of their own.
In 2004, a study published in the Journal of Applied Toxicology (Darbre, P.D., et al., DOI: 10.1002/jat.958) detected parabens (a class of preservative commonly used in personal care products and cosmetics) in human breast tumors. The researchers speculated that parabens, previously shown to have weak estrogenic activity, may migrate into breast tissue from antiperspirants or deodorants applied to the underarm area. This finding sparked widespread media coverage and a rash of email warnings proclaiming that antiperspirants cause breast cancer.
Critics point to several shortcomings of the study that ignited this firestorm. For example, the researchers analyzed a statistically insignificant number of breast tumors and no healthy breast tissue, making it impossible to link parabens with cancer. In addition, the levels of parabens that the scientists detected in their control samples—which contained no biological tissue—were similar to those in tumor samples. Therefore, all of the samples could have been contaminated with parabens from laboratory glassware or lab workers’ cosmetics. Also, most major brands of antiperspirants and deodorants do not contain parabens.
“This deeply flawed paper almost single-handedly caused all the hysteria over the use of parabens that continues today,” says Dene Godfrey, technical sales manager at a major chemical distributor in the United Kingdom and author of a series of articles called “Parabens in Perspective”. The backlash against parabens and other traditional preservatives—scientifically justified or not—has created a substantial market for “natural” preservatives derived from plant sources. However, experts contest whether natural preservatives are truly safer than, not to mention as effective as, their synthetic counterparts. Meanwhile, manufacturers eager to strike a balance between consumer sentiment and scientific fact are increasingly pursuing effective alternatives to traditional preservatives (Table 1).
Curbing microbial growth
Although some factions clamor for “preservative-free” products, most formulators agree that some type of preservative is essential to curb the growth of microorganisms. Because cosmetics have plentiful water and nutrients, as well as a hospitable pH and temperature, products such as lotions, creams, and makeup are potential breeding grounds for microorganisms. Bacteria, yeasts, and molds may be introduced during the manufacture or packaging of a product, or by the consumer during normal use (Fig. 1).

Improperly preserved personal care products can cause skin irritation, infections, blindness, and even serious illness or death. In 2006, five intensive-care patients at a hospital in Barcelona, Spain, contracted life-threatening infections with the bacterium Burkholderia cepacia. Their illnesses were ultimately traced to a moisturizing body milk used in the patients’ care. Researchers detected the bacterium in unopened bottles of the body milk.
Preservatives help maintain product integrity by killing microorganisms. Most preservatives are active against a single type of microbe. For example, parabens are most effective at killing fungi (yeasts and molds), whereas formaldehyde donors (discussed below) target bacteria. “No single preservative is equally effective against all types of microorganisms,” says Philip Geis, principal consultant at Geis Microbiological Services in Gainesville, Florida, USA. “As a result, a mixture of preservatives is often used by formulators to ensure that a broad spectrum of antimicrobial activity exists.”
Cosmetic formulators gauge the efficacy of preservatives with a preservative effectiveness test (PET). They inoculate a product with a mixture of bacteria, yeast, and mold, then measure the levels of the microbes over time, typically 28 days. If the number of microorganisms in the product does not fall to acceptable levels, the product fails the PET and should not go to market.
Despite recent safety concerns, parabens remain the most widely used preservatives in cosmetics, with a history dating back to the 1920s. Chemically speaking, parabens are esters of p-hydroxy benzoic acid (Fig. 2).
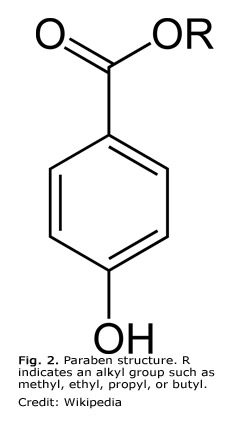
The identity of the alkyl group in the ester (e.g., methyl, ethyl, propyl, or butyl) influences the properties of the paraben. Even at low concentrations, most parabens are extremely effective at controlling the growth of yeasts and molds and are also active against many bacteria. Allergic reactions to parabens are rare, ranging from 0 to 4.2% of the population (Cashman, A. L., and Warshaw, E. M., DOI: 10.2310/6620.2005.05008, 2005). Also contributing to parabens’ long-standing popularity is their antimicrobial activity over a wide pH range, their heat stability, lack of odor and color, and cost-effective synthesis from petrochemicals.
Safety depends on the dose
In the late 1990s, various studies began to raise concerns about parabens. A 1998 paper published in Toxicology and Applied Pharmacology demonstrated that some parabens can weakly bind to estrogen receptors on cells, making parabens possible endocrine-disrupting chemicals (Routledge, E. J., et al., DOI: 10.1006/taap.1998.8544). Known endocrine disruptors, such as DDT and bisphenol A, can cause cancer, birth defects, early puberty, and problems with sexual development in exposed animals.
When the researchers injected parabens under the skin of rats—a more direct route of exposure than for cosmetics applied to the skin’s surface—the parabens activated estrogen receptors only weakly compared with the receptors’ natural ligand, estradiol. In fact, the most potent paraben, butylparaben, was about 100,000 times less effective than estradiol at stimulating uterine growth in sexually immature female rats (a measure of early puberty). The researchers calculated that the lowest dose of butylparaben that caused an adverse effect in rats was 200 mg/kg body weight/day.
To put this finding in perspective, a 60 kg person would need to apply 40 kg of 0.03% butylparaben-containing cosmetics every day to experience the weak estrogenic effect observed in rats, says Godfrey. And this assumes that the skin can absorb 100% of topically applied butylparaben; reported estimates range from 4 to 60%. According to Godfrey, this study, like many that reported adverse effects of parabens, was taken out of context by the media, activist groups, and bloggers who often failed to mention that the tested concentrations of parabens bear no semblance to real-life exposures.
National and international regulatory agencies remain unconvinced of parabens’ health threats. Parabens are approved worldwide for use in personal care products at specified concentrations. According to a US Food and Drug Administration (FDA) fact sheet on parabens, “[The] FDA believes that at the present time there is no reason for consumers to be concerned about the use of cosmetics containing parabens.” Similarly, the European Union (EU) Scientific Committee for Consumer Safety (SCCS) considers methylparaben and ethylparaben safe for use in cosmetics at concentrations up to 0.4%, and propylparaben and butylparaben at maximum total concentrations of 0.19%.
Still, natural cosmetic formulators such as Barbara Olioso, founder of Forest Secrets Skincare, a line of all-natural skincare products based in the United Kingdom (Fig. 3), are concerned about possible long-term effects of parabens. “Just because we lack evidence, doesn’t mean that they are safe,” she says. “There’s a lot of data on rats, but rats can metabolize parabens much better than we can. We need data on humans, and we need to know how much parabens we absorb and what happens once they’re absorbed into the body.”
 Formaldehyde donors, another class of preservative with a long history of use in cosmetics, have likewise attracted the ire of nongovernmental organizations and consumer advocacy groups. These compounds gradually release small amounts of formaldehyde into a product, killing bacteria and fungi. Given that formaldehyde is a well-known toxin, irritant, and carcinogen, it is perhaps understandable why people should object to its use in personal care products.
Formaldehyde donors, another class of preservative with a long history of use in cosmetics, have likewise attracted the ire of nongovernmental organizations and consumer advocacy groups. These compounds gradually release small amounts of formaldehyde into a product, killing bacteria and fungi. Given that formaldehyde is a well-known toxin, irritant, and carcinogen, it is perhaps understandable why people should object to its use in personal care products.
However, according to David Steinberg, a personal care product consultant at Steinberg & Associates, Inc., in Plainsboro, New Jersey, USA, the dose makes the poison. He recalls a discussion with a woman at a scientific meeting who was adamantly opposed to the use of formaldehyde donors in personal care products. “I asked her to take a deep breath and exhale, which she did,” says Steinberg. “I said, ‘Do you realize you just exhaled between five and ten parts per billion formaldehyde, which is what you want to ban? How are you still alive?’” He notes that cells produce formaldehyde during normal metabolic processes.
In 2006, Steinberg founded the Cosmetic Preservatives Council, a consortium of preservative manufacturers that defends and advocates the use of traditional preservatives. “We got together to try to save some of our preservatives from these ridiculous attacks, and we’ve tried to present facts as opposed to emotionalism,” he says.
Nevertheless, some cosmetic companies have succumbed to the public backlash against traditional preservatives. Increasingly, products such as sunscreens prominently display the label “paraben-free” on their packaging. In March 2012, the Estée Lauder Companies (New York, New York, USA) issued a statement on parabens explaining that “as a result of previous conversations with our consumers, we have already begun the process of phasing out the use of parabens, without compromising the integrity of our products”.
For manufacturers seeking alternatives to parabens and formaldehyde donors, blends of methylisothiazolinone and its halogenated derivative methylchloroisothiazolinone have become popular choices. This preservative blend has broad-spectrum activity against bacteria and fungi and is effective at very low concentrations. However, the isothiazolinones, like all preservatives, are not free from controversy: a 2002 report showed that methylisothiazolinone is toxic to cultured neurons (Du, S., B. McLaughlin, S. Pal, and E. Aizenman, J. Neurosci. 22:7408–7416, 2002). Yet, again, critics contend that the concentration of methylisothiazolinone tested in the experiments greatly exceeds exposure levels from cosmetics. According to a response statement issued by the Cosmetic, Toiletry, and Fragrance Association, applying the preservative directly to rat nerve cells in a petri dish in no way resembles exposure to isothiazolines through the skin.
Definitions of “natural”
Consumer sentiment against synthetic preservatives and the allure of “100% natural” labeling have prompted some manufacturers to seek natural alternatives. However, in the minds of consumers and manufacturers alike, the definition of “natural” is often murky at best. “There are different shades of natural,” says Olioso. For the purists, oils and extracts distilled or otherwise physically separated from plant sources are the only acceptable naturals. “Nature-derived” or “hybrid” refers to naturally occurring ingredients that are chemically modified to improve their properties in cosmetics. “Nature-identical” preservatives are identical to those found in nature, but are synthesized in a laboratory. “All of these categories can be called natural, but they have different implications on health and the environment, and different prices, as well,” says Olioso.
Wendy Robbins, marketing manager at From Nature with Love, a wholesale supplier of natural cosmetic ingredients based in Oxford, Connecticut, USA, agrees that the term “natural” is highly subjective. Robbins defines “natural” as “an ingredient derived from natural botanicals and processed in as green a manner as possible—one that retains the ‘wholeness’ of the botanical.” For example, tea tree oil is typically steam distilled from the leaves of the tea tree, Melaleuca alternifolia. “The resulting essential oil is generally classified as a natural preservative because the oil is left intact with its balanced ratio of naturally occurring constituents,” says Robbins. Scientists have traced the antimicrobial properties of tea tree oil to a mixture of terpenes: chemicals with variable numbers of linked isoprene units.
“Natural” is not “organic”
Many people confuse “natural” with “organic,” although the two terms are not interchangeable. The term “natural” is not regulated in the United States, but most international regulatory agencies define natural ingredients similarly to the European Union’s Registration, Evaluation, Authorisation and Restriction of Chemical Substances (REACH) regulations: “ . . . a naturally occurring substance, unprocessed or processed only by manual, mechanical, or gravitational means, by dissolution in water, by flotation, by extraction with water, by steam distillation, or by heating solely to remove water, or is extracted from air by any means.” In principle, products labeled “all natural” or “100% natural” should only contain ingredients that comply with this definition.
In contrast, “organic” refers to ingredients produced through organic agricultural practices, without the use of fertilizers or pesticides. The US Department of Agriculture (USDA) label “organic” indicates products that contain at least 95% organically produced ingredients. This distinction between natural and organic means that a personal care product could be 100% natural and not organic, and vice versa. Manufacturers can have their products designated natural or organic by international certifying agencies such as Ecocert and Natrue, but the requirements for certification vary, sometimes substantially, with the certifying body.
Grapefruit seed extract is a liquid derived from the seeds, pulp, and white membranes of grapefruit. Although marketed by suppliers as an effective natural preservative, in 1999 researchers discovered that many commercially available grapefruit seed extracts were contaminated with synthetic preservatives such as parabens, triclosan, and benzethonium chloride (von Woedtke, T., et al., Pharmazie 54:452–456). The researchers reported that in the absence of these synthetic preservatives, grapefruit seed extracts had no antimicrobial activity. However, Robbins maintains that “our grapefruit seed extract is paraben-free, unlike many other products being marketed as grapefruit seed extract. The efficacy has been tested against gram-positive and -negative bacteria and fungi, and it has performed exceptionally well.”
Going natural has its own challenges
A challenge of working with essential oils and extracts is that the same product obtained from different suppliers, or even different batches of a product from the same supplier, can vary considerably in composition. Factors such as the season of harvest, region of cultivation, plant parts used, and type of processing can affect the levels of various chemicals in botanical oils and extracts (Fig. 4). In addition, botanicals can be contaminated with pesticides or mycotoxins. Essential oils and extracts must often be used at high concentrations (up to 2% of a formula) to be effective preservatives, which can confer unpleasant colors, odors, or textures to personal care products.

Even at these high concentrations, most natural preservatives are not as effective as traditional synthetic preservatives. “Natural substances that show antimicrobial activity are usually not adequate for broad-spectrum preservation,” says Robbins. “Natural antimicrobials and natural antioxidants can be combined to protect and extend the shelf life of natural products, but they will not offer the extended shelf life that traditional preservatives offer.” Although antioxidants such as tocopherols (vitamin E) and ascorbic acid (vitamin C) don’t actually kill bacteria, they can help keep oils in personal care products from going rancid, reducing the potential for microbial growth.
While consumers tend to view natural products such as tea tree oil and grapefruit seed extract as more benign than unpronounceable chemicals made in a lab, Steinberg notes that the naturals have not been as extensively tested for safety as synthetic preservatives. “People would much rather use something labeled as natural that has virtually no safety testing and no ability to be reproducible than traditional preservatives that have been safety tested for decades, evaluated by independent peer scientists, and approved by regulatory agencies worldwide,” he says.
Olioso argues, “We have evolved with naturals, so in theory they’ve been tested for much longer, but in a different way. The data haven’t been presented to scientific committees and papers haven’t been published, but our bodies are more familiar with them.” Nevertheless, just as for synthetic preservatives, some studies have reported adverse effects of natural preservatives, including allergic reactions, early puberty, and cancer. And just like the warnings against synthetics, Olioso says that many of these studies have been taken out of context to unfairly condemn natural preservatives.
Nature identicals offer a middle ground
Godfrey disputes the assumption that natural products are inherently safer and gentler than synthetics. He notes that some of the deadliest toxins on earth are produced naturally by plants and animals, including tetrodotoxin from the pufferfish, ricin from castor beans, and venom from the King Cobra. “It is the height of human arrogance to believe that nature exists purely for the benefit of humans,” he says. “Every individual species exists for its own sake. If something produced within a plant happens to be beneficial for humans, that’s pure coincidence”—and not evidence of Mother Nature’s benevolence toward our species.
However, consumers’ infatuation with naturals is unlikely to fade anytime soon. So some natural cosmetic formulators choose to work with nature-identical preservatives as a middle ground between traditional preservatives and botanical oils and extracts. “I’m quite in favor of nature identicals because they are cost effective, they work, and they’re the same molecular structure as found in nature,” says Olioso. An example of a nature-identical preservative is benzoic acid, which is found in apples but also commercially synthesized. Benzoic acid is most effective against yeast and mold at pH 4, limiting its use to acidic products such as facial peels.
Because some edible botanicals contain parabens, the controversial preservatives could be considered nature-identical. However, few if any natural product aficionados recognize parabens as such. Some consumers worry about trace contaminants derived from the petrochemical sources of commercial parabens. In addition, “Concerns exist over how our bodies process synthetic parabens in contrast to parabens found naturally in foods like cucumbers and mangos,” says Robbins.
Godfrey disagrees that synthetic preservatives such as parabens pose higher risks than the same preservatives isolated from natural sources. “The body can’t distinguish between different sources of the same molecule,” he says.
”Natural” can be expensive
Nonetheless, in the minds of many consumers, preservatives extracted from plants are preferable to those made in factories. Yet because plants usually contain only minuscule (parts per million) amounts of preservatives and other cosmetic ingredients, isolating the chemicals can be cost prohibitive. Back in 1983, Steinberg worked at a chemical manufacturing plant. He recalls that a customer wanted to buy 1 kilogram of the cosmetic ingredient allantoin extracted from comfrey root. “I told him he would have to send me a certified check for 15 million dollars, and I would get him one kilo of naturally obtained allantoin from comfrey root,” says Steinberg. “The guy went nuts, and I said, ‘Do you know how many millions of pounds of comfrey root I would have to buy?’” Steinberg notes that at that time, the price of one kilogram of synthetic allantoin was $4.00. “You’ll never guess what he bought,” he says.
Because none of the preservatives currently on the market appeals to everybody, one might expect that developing new preservatives would be fertile ground for research and development. However, Steinberg notes that no truly new preservatives have been introduced for years. “The last preservative that was approved worldwide and is still available [methylisothiazolinone] cost the manufacturer in excess of ten million dollars in safety testing,” says Steinberg. “That’s a major deterrent for introducing new preservatives.”
The advantages of blends
As a result, most of the innovation in the preservative industry has come from developing new blends of preservatives. Blends offer broader-spectrum microbial coverage and allow formulators to use lower concentrations of each preservative in the blend, improving safety. Nipaguard® POM, a broad-spectrum preservative blend from Clariant , contains 80% phenoxyethanol, 15% methylparaben, and 5% piroctone olamine. On the natural side, Bio-Botanica offers Suprapein™, a blend of oregano, thyme, cinnamon, olive, rosemary, peppermint, lavender, goldenseal, and lemon extracts with broad-spectrum antimicrobial activity. Striving for the best of both worlds, RITA Corp.’s Ritative SR4L Magnolia combines Magnolia acuminata bark extract with the nature-identical preservatives phenoxyethanol and glyceryl caprylate.
The holy grail for personal care product manufacturers and consumers would be a preservative-free system that maintains product integrity. A number of manufacturers have used a loophole in Annex VI of the EU Cosmetics Directive to make deceptive “preservative-free” claims. Annex VI is a list of preservatives permitted in cosmetics sold in the EU. Some fragrances and other chemicals have preservative activity, yet are not listed on Annex VI. The loophole allows manufacturers to include these preservatives in products labeled “preservative-free,” if they claim that the chemicals are being used for purposes other than preservation.
True preservative-free personal care products are rare. Homemade or artisanal products lacking preservatives require refrigeration and have extremely short shelf lives (2–4 weeks). For some products, formulators can use the concept of water activity to reduce preservative requirements. Water activity is a measure of the water in a product that is available for microbial growth. Formulators can calculate water activity by measuring the partial vapor pressure or dew point of a product. On a scale of 1.0 (pure water) to 0 (completely dry), a water activity of 0.7 is usually sufficient to prevent the growth of microorganisms. Typical water activities for personal care products range from 0.86 (hand cream) to 0.98 (hand lotion).
Water activity and packaging
Many cosmetic formulators routinely measure the water activity of new formulations to get an idea of what preservatives they need to add. “The water activity gives us a roadmap,” explains Steinberg. “Let’s say we determine the water activity is 0.85. At 0.85, no yeast or bacteria will grow in the product, so the only thing we need to worry about is mold.” Formulators can add molecules like salts and glycols to lower a product’s water activity. However, products with water activities below 0.7, therefore requiring no preservatives, are usually aesthetically unpleasing.
Careful consideration of packaging can also reduce, but not eliminate, the requirement for preservatives. A facial cream in a bottle with a pump is less likely to be contaminated with microbes from a consumer’s fingers than the same product in a wide-mouth jar, says Geis.
Although synthetic preservatives bear the brunt of criticism from anti-preservative groups, natural preservatives are not free from controversy. “Methyl eugenol in rose oil is an absolutely amazing preservative,” says Olioso. ”But because tests in rats showed carcinogenic effects on the liver, rose oil has been restricted to the point that it’s almost impossible to use. The maximal allowed concentration of methyl eugenol in leave-on products is only 0.0003%.” She thinks that manufacturers of natural ingredients should form an organization in which they can share safety data on naturals, split costs of safety tests, and advocate for natural ingredients, similar to what Steinberg’s Cosmetic Preservatives Council does for synthetic preservatives. “Because natural products are not patented, who is going to invest in extensive safety tests?” asks Olioso. “There has to be some kind of movement to safeguard the naturals and put things into perspective.”
Currently, there are no signs of a cease-fire in the campaign against preservatives. “You have activist groups who believe that there is no such thing as a good preservative, and that we can’t have preservatives, and we shouldn’t have cosmetics,” says Steinberg. Defenders of synthetic and natural preservatives can perhaps unite in the hope that at the end of the preservative wars, microbes don’t emerge as the winners.
Laura Cassiday is a freelance science writer and editor based in Hudson, Colorado, USA. She has a Ph.D. in biochemistry from the Mayo Graduate School and can be contacted at lauracassiday@yahoo.com.
