Biotechnology conquers consumer goods
By Rebecca Guenard
June 2019
- A diverse set of products is entering the consumer goods market thanks to the successful application of biotechnology.
- Manufacturers are routinely editing genes to investigate the viability of synthesizing a cellular product that will improve or replace the components used in automobiles, food, textiles, personal care, home care, and other products.
- The technology has become economically competitive by developing high-throughput testing and finding opportunity in the products consumers use every day.
The world of biotech manufacturing is comprised of start-ups and strongholds. Companies that have manufactured consumer goods for over 200 years innovate alongside businesses that popped-up just over two years ago. Since 2014, investment in biotech has risen (http://tinyurl.com/y5fythht), and the industry is poised to breach the boundaries of its initial success in the pharmaceutical sector and enter a new realm. “In the healthcare segment, people have seen the value and the power of a technology that can solve and cure diseases. That could never have been done before,” says Michael Arbige, vice president of research and development at DuPont Industrial Biosciences in Wilmington, Delaware, USA. “What we are doing is just an extension of those same tools and those same capabilities applied to very important industrial problems, problems that affect the world every day.” Having reined in the lengthy product development process that once plagued progress, biotechnology has become economically viable for application in everyday products. High-throughput screening of microbial alterations enables researchers to tackle reverse-engineering problems in short order. Ironing out sequencing wrinkles quickly frees up time for optimizing full-scale manufacturing processes. For some products, the improved efficiency of biotech manufacturing coupled with the ability of biotech to fine-tune a molecule‘s specifications gives them a competitive edge in the market. Many consumers and product developers are also making naturally derived materials a priority, and it is obvious that the current market is ripe for the success of biotech in consumer goods. This article looks at how two very different companies use this technology to find success in the market.
A size-dependent approach
When DuPont established its Industrial Biosciences division in 2011, the company had already spent 30 years in the research and development of marketable biotech products (http://biosciences.dupont.com/). During that time, the company also acquired many of the early biotech start-ups, gaining manufacturing facilities around the world. Having teams of scientists hone the biotech manufacturing process over decades has advantages.
Arbige says DuPont Industrial Biosciences commercializes over 50 products a year. They work on problems in animal nutrition, food and beverages, personal care, fabric and home care, and textile processing, to name a few of the industries they serve. “We have a reputation that is very powerful out there and allows us to get into partnerships and gain access to different customers. It opens lots of doors,” Arbige says.
He acknowledges that having the capital backing provided by a large corporation means researchers can gamble on new ideas that may fail. He says that by attempting new things over the years and quickly recovering from disappointment DuPont has built a turn-key biomanufacturing system. According to Arbige, DuPont has put a lot of science into developing a fast, robust process to go from finding a new molecule to manufacturing it at production scale. And, he says, this can be done for any of their product areas.
“One of the things which is foundational to the biotech effort at DuPont is to manipulate microbes to make products better, cheaper, and more efficiently across a range of hosts—and for a range of products,” Arbige says. Despite millions in investment dollars flowing into new biotech start-ups, they do not have the infrastructure to support this type of approach to product development (http://tinyurl.com/y5fythht). That means small companies have the best chance at success with a product that fills a niche.
“When you are a small company like us, you can't explore too many things at one time,” says Scott Franklin, co-founder and chief scientific officer at Checkerspot, a materials biotech start-up in Berkeley, California, USA. As a former vice president of Solazyme, which filed for bankruptcy in 2017, after unsuccessful attempts at economical production of first biofuels then food oils, Franklin says he has learned from the mistakes of the early days.
Checkerspot’s objective is to manufacture products with improved performance and better environmental sustainability than those that are petrochemically derived. They have partnered with textile and outdoor gear companies to help them create moisture-wicking coatings for T-shirts, and they are working on polyurethane composites for a variety of applications, like skis and surfboards. They address these different applications by optimizing triglycerides for conversion to chemically versatile starting materials, such as polyols.
“We are very deliberate about developing a polyol, getting it into a material, and getting that material into our brand to animate what we can do with our technology,” Franklin says. Bioengineered microalgae are central to this process.
Microalgae, seeds of change
Algae have been considered a potential source of oleochemicals for a long time due to their ability to produce large amounts of oil on fewer acres than typical oilseed crops. That is especially true for certain types of microalgae that can be raised on sugar inside a fermentation tank. Checkerspot further expands the organisms’ utility by genetically modifying their DNA so the microalgae bulge with triglycerides containing purposefully designed chemistry.
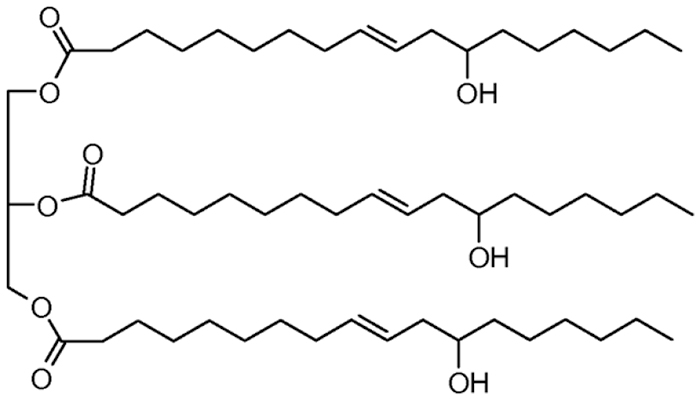
Some seed oils, like castor, naturally produce a fatty acid with a hydroxyl group that when reacted with an isocyanate creates a urethane linkage. Other oil seeds contain fatty acids with points of unsaturation that can be converted into hydroxyl groups for the same purpose. Neither type of plant oil is ideal for synthesizing a polyurethane, according to Franklin, because the Natural Oil Polyols (NOPs) are all 18 carbons long with chemical functionality in a limited number of locations. When the urethane is polymerized, the fatty acyl dangles from the chain often imparting an undesirable elasticity to the final polyurethane material.
FIG. 1. Structure of castor oil triglyceride(Liu, Zhu, RSC Green Chem, 2015). (Available via license: CC BY 4.0.)
At Checkerspot, scientists are manipulating the cellular activity of the microalgae to produce fatty acids with a variety of chain lengths and with functionality in multiple locations. “When we think about engineering triglycerides—as it relates to making polyols and using those for polyurethanes—what we are trying to get to and explore is outside the narrow range that exists today,” Franklin says.
As is typical of biotech companies, nature inspires the innovations at Checkerspot. They searched for plants that produce seed oil containing shorter fatty acid chains. They found a plant that produces seed oil with fatty acid chains of 10 and 12 carbons that contain double bonds and hydroxyl groups throughout. By studying the biosynthesis of the oil, Franklin and his team can usurp the plant’s techniques and bioengineer those into their microalgae.
“If we have one little piece of that oil biosynthetic machinery that we can focus on, like a group of enzymes, then we can understand how to manipulate it,” Franklin explains. “So instead of chains 18 carbons long, can we put double bonds on chains that are ten carbons long? And if we put them on chains that are ten carbons can we put them at different positions?”
He says they have successfully engineered the enzymes within their microalgae to install a double bond at a precise location in the carbon chain. These changes have a direct impact on the properties of the polyurethane that will ultimately be the final product. By this means, Checkerspot can make coatings, adhesives, foams, resins or elastomers from a sustainable source that are nothing like what is available from oleochemicals currently on the market.
“When you start to combine all this technology, the universe of oleochemicals gets very, very big now,” says Franklin. He says Checkerspot is interested in making renewable lubricants and renewable dielectric fluids that have properties that are not possible with the currently available triglycerides.
While the company explores the capabilities of their bioengineered organisms to synthesize new triglycerides, they maintain a focus on the development and formulation of their current product for outdoor gear. Franklin says they have an organism that makes an oil at an impressively high percentage of the dry cell weight, meaning most of the cell is a triglyceride. They have built a foundation of experience making polyols from that triglyceride and formulating it into polyurethanes designed for specific applications. He says as this product comes online, he looks forward to applying all Checkerspot has learned to developing their next product. “There is not enough time in my lifetime to explore all the possibilities of all the potential materials that we can make,” Franklin says.
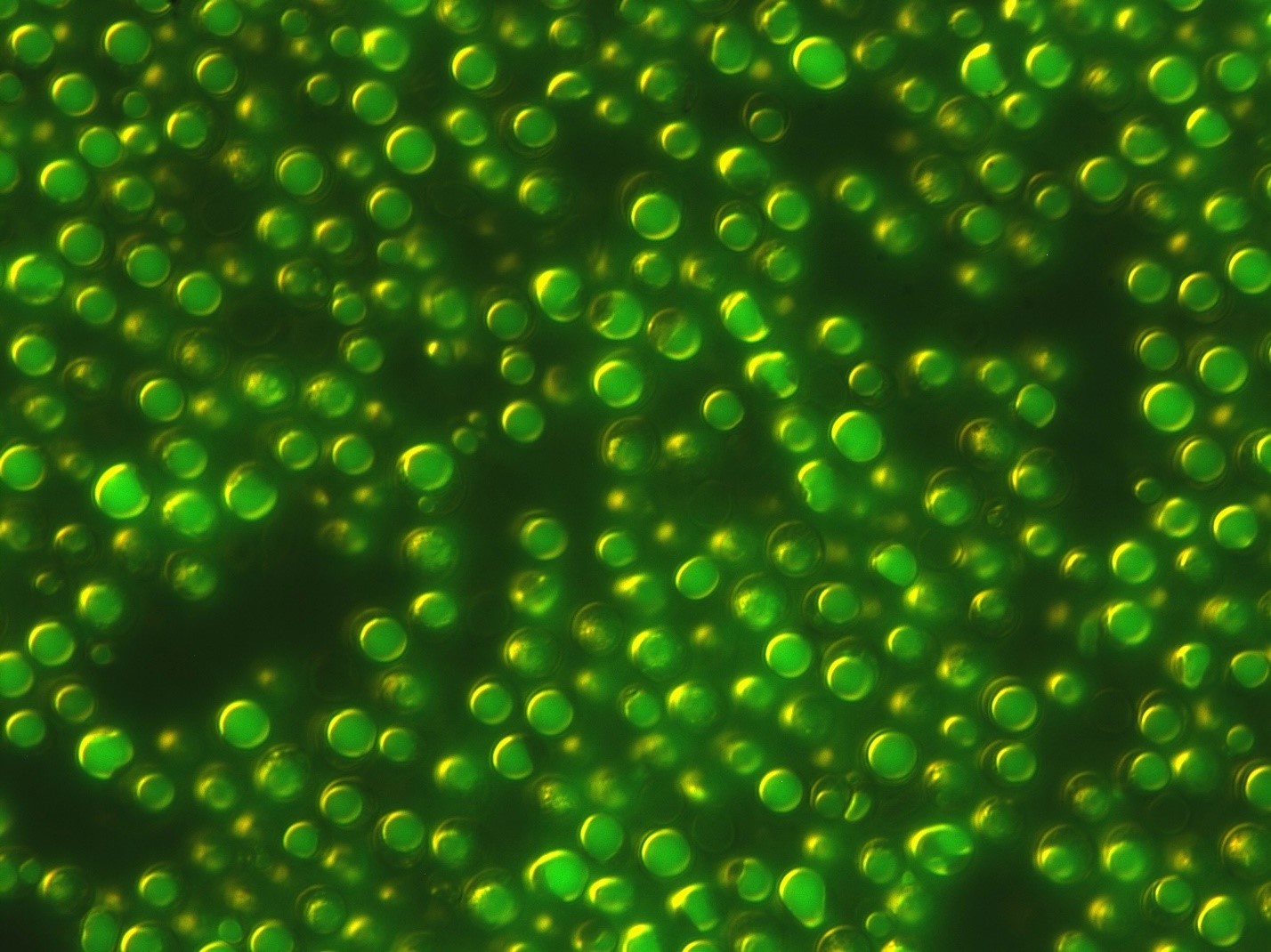
FIG. 2. A quarter of the width of a human hair, these oil-engorged cells have been stained with BODIPY, a fluorescent lipophilic dye that highlights the large lipid droplets inside each cell. This fluorescent image is superimposed over a light microscope image to show how much of each cell's total volume is taken up by oil.(Credit: Carolina Bagnota for Checkerspot)
FIG. 3. On the left, dried algae biomass. On the right, purified triglyceride algae oil.(Credit: Xan Marshland for Checkerspot)
Enzymes, enzymes everywhere
Unlike a start-up, a company like DuPont has the liberty to explore multiple biotech product applications in tandem. In addition, established partnerships mean there is no shortage of problems to solve. “For example, we will go to Procter & Gamble (P&G) and ask, ‘What are your big issues that you see coming up?’” Arbige says.
When DuPont posed that question in 2012, P&G responded that they were interested in offering customers a more environmentally sustainable way to wash clothes. They asked DuPont to help them create a cold-water detergent. Arbige enlisted the Industrial Biosciences enzyme group to address the task.
“We have a whole class of materials that we make called enzymes which affect different industries such as the food industry, the fabric and household care industry, the animal nutrition industry, the personal care industry,” says Arbige. “Enzymes can breakdown or synthesize a wide range of the natural materials that are out there: starches, fats and oils, proteins, and all the cellulosic materials.”
For a detergent to work in cold water, the DuPont scientists knew that they would need to find an enzyme that could break down stains at low temperatures. Like Checkerspot, they turned to nature to find an organism that was already performing this task. “We go to cold-climate countries or the tops of mountains, and we find new microbes in these environments,” Arbige says. “We find the enzymes that are in those microbes, and then we have a starting place.”
Arbige adds that since they were interested in breaking down protein stains, in this instance they looked for a cold-water protease. He says, typically once they find an enzyme it is rarely close to operating as a product. The enzyme must function and be stable alongside other chemistries that are in a detergent, such as surfactants, builders, and chlorinating compounds. In addition, the detergent needs to work in various regions around the world and be effective in a range of water chemistry.
“With all these parameters in mind, we use protein engineering to change amino acids in the enzyme to other amino acids that give it new functionality in the detergent,” Arbige says. “We will modify that protein and study it under the conditions that we have designed to see if it works under those conditions.” He says the enzyme evolves over months to years before they begin to determine if they can make it economically by producing it through fermentation in microbes. Finally, after formulating it to work in their customer’s product the enzyme is commercialized.
Arbige says DuPont makes enzymes that are a sustainably sourced from natural ingredients, which can also improve a food’s nutritional value and reduce food waste. Typical emulsification compounds, for example, do not fit the demands of a clean label. DuPont produced an enzyme that works in-situ as an emulsifier to stabilize the interface between oil and water. In addition, they have created enzymes that extend the shelf-life of bread by breaking down certain starches that would cause the bread to go stale. Their most recent bioengineered enzyme product was designed to be an ingredient in yogurt. The enzyme converts sugar in yogurt into a prebiotic fiber that helps with digestion.
To keep bringing new biotech products to market, Arbige says you must maintain the innovative mindset of a small start-up despite being a big company. Failure happens all the time, and you cannot be afraid of it, he says, recover and move on quickly.
The high-throughput advantage
New biotechnology tools help with that speedy turnaround. Arbige says that for many of DuPont’s products, these tools make it possible to go from concept to production in little over a year. Characterization tools such as genome sequencing, transcriptome analysis, and metabolic analysis, have become faster and cheaper. This means scientists can see the outcome of their reverse-engineering efforts within hours.
The improved speed of computing means that Arbige and Franklin can search large databases of gene sequences for the natural species they model their engineered organisms on. This allows them to get to the starting line faster. Once there, they can go through a rapid succession of fine-tuning the organism and testing its metabolic pathways.
“We can expend a lot of effort doing molecular genetics and molecular biology on the organism to start to generate molecules that will be interesting,” Franklin says. “On the front end, as we are doing the work in the organism, the cost is incremental.” He adds that if they have an idea about engineering unsaturation into a triglyceride, they can test if their idea was successful without having to ferment a large volume of microalgae.
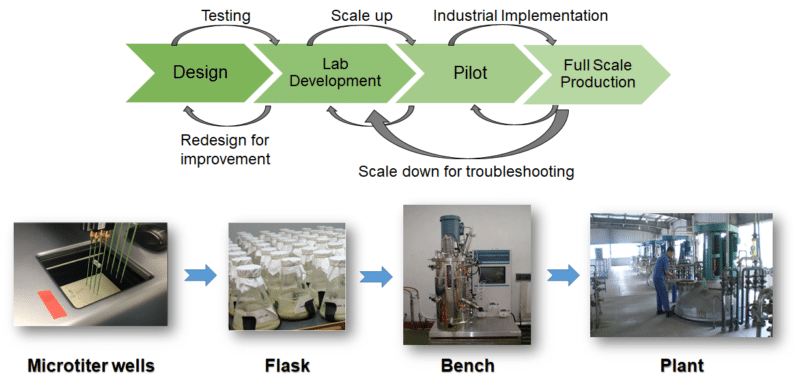
FIG. 4. Conagen’s integrated manufacturing chain.(Credit: Conagen Inc.)
Biotech companies have more options for product development as new businesses launch to serve a role once available only through government-funded programs. The Advance Biofuels and Bioproducts, Process Development Unit (http://abpdu.lbl.gov/about-us) is a US Department of Energy-funded laboratory operating as part of Lawrence Berkeley National Labs in Emeryville, California, USA. The facility opened in 2012 to assist biotechnology researchers who were interested in developing a microbe to produce at full-scale. Privately run companies such as Conagen, Inc. (http://conagen-inc.com) and Cultural Biosciences (https://www.culturebiosciences.com) are now establishing themselves to serve the same purpose. With the number of synthetic biology start-ups increasing, fermentation space is at a premium, and these companies offer start-ups a chance to prove themselves to investors.
As more support industries become part of the infrastructure of biotechnology, the number of naturally derived consumer goods will grow. The industry has expanded beyond its original objective of replacing fossil fuels and into lower-volume, higher-value products that are more likely to bring economic success as a component of consumer goods. Everything from pet food to anti-aging cream to indoor carpet now has a biosynthesized option. Today, with a microbe, a gene-editing tool, and a computer algorithm, scientists can make any product they can imagine.
Rebecca Guenard is the associate editor of Inform at AOCS. She can be contacted at rebecca.guenard@aocs.org.
References
- Polyols and polyurethanes from crude algal oil, Petrovic, Z.S., et al., J. Am. Oil Chem. Soc. 90: 1073–1078, 2013.
- Biological oils as precursors to novel polymeric materials, Petrović, Z.S., I. Javni, and M. Ionescu, J. Renew. Mater. 1: 167–186, 2013.
- Biotechnology for Biofuel Production and Optimization, Eckert and Trinh, Elsevier, Cambridge, MA, 2016.
- Heterotrophic cultures of microalgae: metabolism and potential products, Bashan, Y., et al., Waters Research 45: 11–36, 2011.
Biotech lipids for sun protection
To solve a problem with biotechnology, researchers often turn to nature to find a living organism that functions within the specific parameters of the problem as part of its daily existence. One group of organisms, haptophyte microalgae, may hold the solution to prevent coral reefs from dying.
In 2018, the state of Hawaii issued a ban on sunscreens containing the organic ultraviolet (UV) filters, octinoxate and oxybenzone (https://www.capitol.hawaii.gov). A year later the city of Key West, Florida, USA, did the same (https://tinyurl.com/ycmlx7r8). Now, Miami, Florida is considering a similar measure (https://tinyurl.com/yyyr6qb9). The ban of these sunscreen ingredients results from research showing that the compounds are causing coral reefs to sicken and die (https://doi.org/10.1289/ehp.10966). The US National Parks Service reports that 4,000 to 6,000 tons of sunscreen is washed from human skin into the ocean yearly.
While saving coral reefs is a priority for many consumers, so is avoiding skin damage from the sun’s UV rays. Sunscreen formulators need alternative filters that satisfy both concerns. A team of researchers investigated a family of lipids known as alkenones and found them superior to the banded filters when blocking UV light (https://doi.org/10.3390/cosmetics6010011).
Gregory O’Neil, organic chemistry professor at Western Washington University in Bellingham, Washington, says that alkenones are well-known compounds that still need in-depth study to understand how they can be used in personal care applications. “The history of these alkenones is really fascinating,” O’Neil says. “But nobody has really focused on bioengineering or even the physiological role of these compounds.”
In addition to other specialists, O’Neil teamed up with Gabriella Baki, assistant professor of pharmaceutics at the University of Toledo in Toledo, Ohio, USA. Baki formulated the lipids into sunscreen and lipstick. While the ingredient functioned well in these products, it did not thicken the sunscreen adequately enough for spreading.
According to O’Neil, their findings indicate that more research on these compounds could prove valuable, especially if biotechnology were applied. He says little is known about the biosynthesis of alkenones by algae. Learning more about how organisms make the compounds could guide future research to bioengineer alkenones to act as naturally derived UV filters. “It would be nice to know how you might tweak the organism to change the alkenone profile,” says O’Neil. “Or, in our case, we would be interested in learning how to increase alkenone production within the algae.”
References
- Alkenones, a renewably sourced, biobased wax as an SPF booster for organic sunscreens, Baki, G., et al., Cosmetics 6: 11, 2019.
- Sunscreens cause coral bleaching by promoting viral infections, Pusceddu, A., Environ. Health Perspect. 116: 4, 2008.