Using direct solid phase extraction to analyze persistent organic pollutants in oily food samples
By Katherine K. Stenerson
September 2015
- Many persistent organic pollutants (PoPs) such as dioxins, polychlorinated biphenyls (PcBs), chlorinated pesticides, and polyaromatic hydrocarbons (PAHs), are lipophilic and can consequently end up in vegetable oils and animal-based foods.
- conventional analysis of PoPs in oily food samples involves laborious extraction and cleanup techniques such as liquid/liquid extraction, column chromatography, and gel permeation chromatography.
- the following article describes a new direct solid phase extraction method that can extract nonpolar contaminants from oily samples easily, quickly, and with good recoveries and reproducibility.
Persistent organic pollutants (POPs) are compounds not easily degraded by chemical, biological, or photolytic processes. Therefore, they persist in the environment for long periods of time. POPs encompass harmful man-made compound classes such as dioxins, polychlorinated biphenyls (PCBs), chlorinated pesticides, and polyaromatic hydrocarbons (PAHs). many of these compounds are lipophilic, and accumulate in the fatty tissues of living organisms. The concentrations of POPs increase as they are passed from one organism to another while moving up the food chain (1). Consequently, these compounds can end up in animal-based foods consumed by humans, such as fish, meat, and milk. In the case of plantbased foods such as edible oils, environmental exposure during growing, storage, and/or production can result in POP contamination.
|
General structure for a PCB |
Benzo[a]pyrene |
Concern over exposure to these compounds has led to regulation by some countries limiting levels of certain POPs in foodstuffs. For example, maximum PAH levels in edible oils are designated under European Union (EU) Commission Regulation No. 835/2011, and in edible vegetable oils under the National Standard of the People’s Republic of China (2,3). PCB contamination can be an issue in fish oil, which is a popular dietary supplement, resulting in maximum limits set through EU regulations, and inclusion on the California Proposition 65 contaminant list (4,5).
Traditional approaches for the analysis of POPs in oily food samples often involve an extraction or dilution with organic solvent followed by cleanup with either gel permeation chromatography (GPC) or adsorption chromatography using silica gel, Florisil®, or alumina. Due to the high fat content of oily foods, these aggressive cleanup techniques are required to produce an extract that is clean enough for a robust chromatographic analysis.
Our research group at Sigma-Aldrich/Supelco recently investigated a new approach in which we used a dual layer solid phase extraction (SPE) cartridge to extract POPs directly from vegetable- and animal-based oily food samples. This cartridge, known under the name Supelclean™ EZ-POP NP, consists of two separate sorbent layers. The sorbents used in the cartridge were chosen for their ability to retain the various constituents of fat. The upper layer contains Florisil, which retains compounds containing polar functional groups such as fatty acids. The lower layer consists of a blend of Z-Sep and C18 sorbents. Z-Sep is a zirconia-coated silica, and acts as a strong Lewis acid, thus retaining compounds that are electron donors (Lewis bases). This Lewis acid-base interaction enables the material to retain phospholipids, fatty acids, and mono- and diacylglycerols. The C18 retains triglycerides and other compounds by hydrophobic interaction.
Experimental details
Samples of soybean and fish oils (cod liver) were purchased locally. Soybean oil was spiked at 10 ng/g with the mixture of PAHs listed in Table 1.
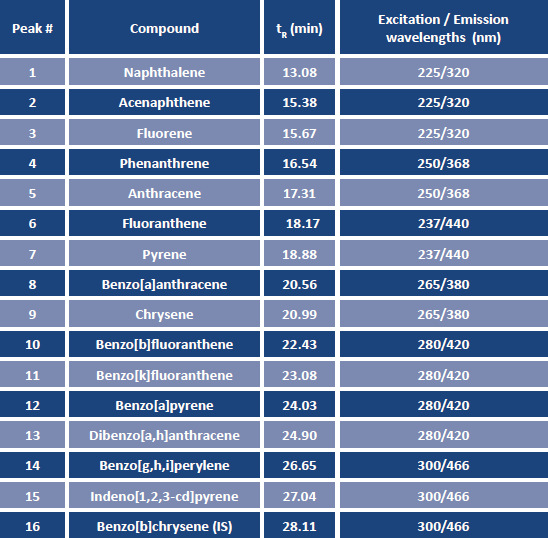
TABLE 1. Acquisition and detection parameters for the selected PAHs analyzed by HPLC-FLD.
These PAHs are included on the US EPA Target Compound List (6). Fish oil was spiked at 10 ng/g with the PCB congeners listed in Table 2.
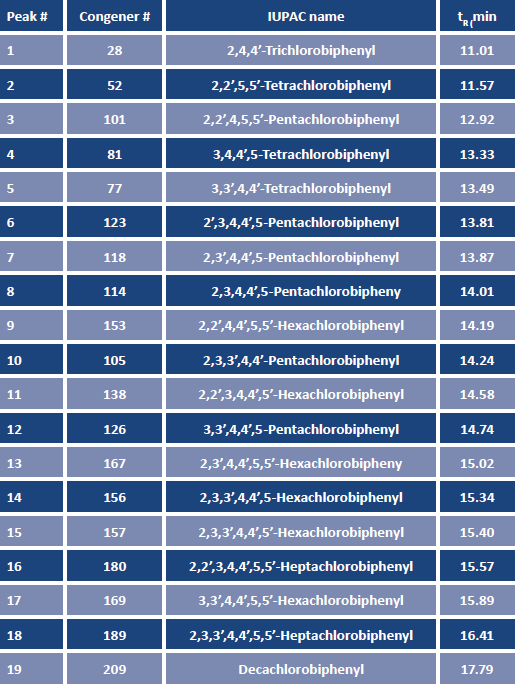
TABLE 2. Detection parameters for the selected PCB congeners analyzed by GC-ECD.
These 19 PCB congeners range from mono- to decachlorinated, and include the 12 coplanar species designated by the World Health Organization (WHO) as "dioxin-like"(7). Samples were extracted using the EZ-POP NP cartridge, following the procedures outlined in Table 3.
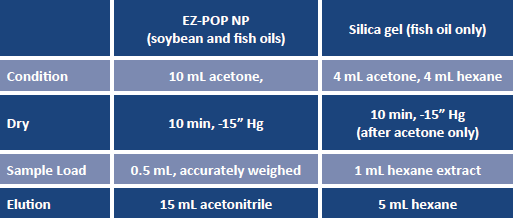
TABLE 3. SPE procedures, soybean and fish oils.
Undiluted oil sample was weighed directly onto the EZ-POP NP cartridge. Figure 1 illustrates the cartridge in use during the elution step with acetonitrile.
 |
| FIG. 1. Dual layer SPE cartridge in use with edible oil sample; extraction of PAHs. The dual layer SPE cartridge contains Florisil (top bed) and a mixture of C18 and zirconia coated silica, commercially available as "Z-Sep" (bottom bed). This combination of sorbents provides multiple mechanisms for retaining fats; adsorption of polar analytes in the case of Florisil, hydrophobic interaction for the C18, and Lewis acid/base interactions for the Z-Sep. Together, these interactions can retain different constituents of fats such as fatty acids and acylglycerols (mono, di, and tri). |
For the analysis of PAHs in soybean oil, an internal standard, benzo[b]chrysene, was added directly to the cartridge after sample loading. Spiked soybean oil samples were analyzed in triplicate, and duplicate samples of the unspiked oil were analyzed for comparison. After elution, the soybean oil eluents were concentrated to a final volume of 0.5 mL. Analysis of the extracts was done by high-performance liquid chromatography (HPLC) using a 25 cm × 4.6 mm ID, 5 μm particle size Supelcosil™ LC-PAH column with a water/acetonitrile gradient. The gradient started at 40% acetonitrile, holding 5 min, then increased to 100% acetonitrile over 15 min, holding at 100% for 12 min. The injection volume was 20 μL, and the column temperature was held at 30 °C. Detection was done by fluorescence, with emission and excitation wavelengths programmed for optimum sensitivity, as described in Table 1.
For the analysis of PCBs in fish oil, no internal standard was used. Replicates of spiked samples (n=6) were prepared, along with triplicate samples of unspiked fish oil. After elution with acetonitrile, the eluents were extracted with 20 mL of hexane, and concentrated to 1 mL. A second cleanup using silica gel SPE was then done using a 1 mL/300 mg SupelcleanTM LC-Si SPE cartridge, following the procedure in Table 3. The final hexane extracts were concentrated to 1 mL for analysis. Analysis of the fish oil extracts was done by gas chromatography (GC) with an electron capture detector (ECD) using a 20 m × 0.18 mm ID, 0.18 μm SLB®-5ms capillary column. The GC oven conditions were: 75 °C, hold for 1 min, ramp at 12 °C/min to 340 °C and hold for 20 min. The injection port temperature was 250°C, and detector temperature was 340°C. A 1 μL sample injection was done in splitless mode, with the splitter opening at 0.75 min. The carrier gas was hydrogen, used at a 1.2 mL/min constant flow rate.
Quantitation of both PAHs and PCBs was done against 5-point calibration curves prepared in the same solvent as the final sample extracts. Responses of the PAHs were calculated in each sample relative to the internal standard. PCBs were quantitated directly from the calibration curve using absolute response.
PAHs in soybean oil
The extract generated from soybean oil using the EZ-POP NP method was fairly clean of background (Fig. 2).
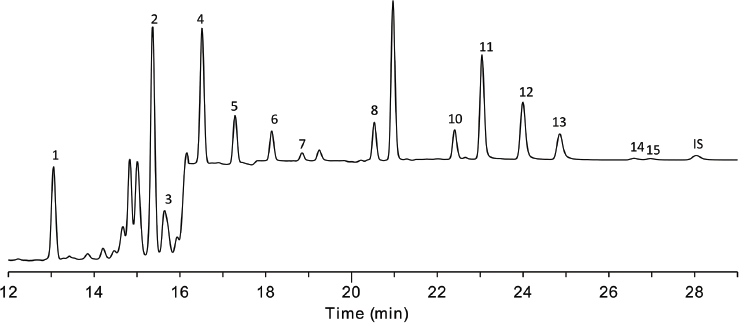
FIG. 2. HPLC-FLD analysis of PAHs in soybean oil spiked at 10 ng/g. Identities of PAH peaks: (1) naphthalene; (2) acenaphthene; (3) fluorene matrix peak; (4) phenanthrene; (5) anthracene; (6) fluoranthene; (7) pyrene; (8) benzo(a)anthracene; (9) chrysene; (10) benzo(b)fluoranthene; (11) benzo(k)fluoranthene; (12) benzo(a)pyrene; (13) dibenzo(a,h)anthracene; (14) benzo(g,h,i)peryelene; (15) indeno(1,2,3-cd)pyrene; (16) benzo(b)chrysene (I.S.)
One problematic matrix peak was present, and it coeluted with fluorene, preventing quantitation of this compound in the spiked samples. All other PAHs were detected free of background. The average recoveries obtained for the 3 spiked replicates of soybean oil are presented in Table 4.
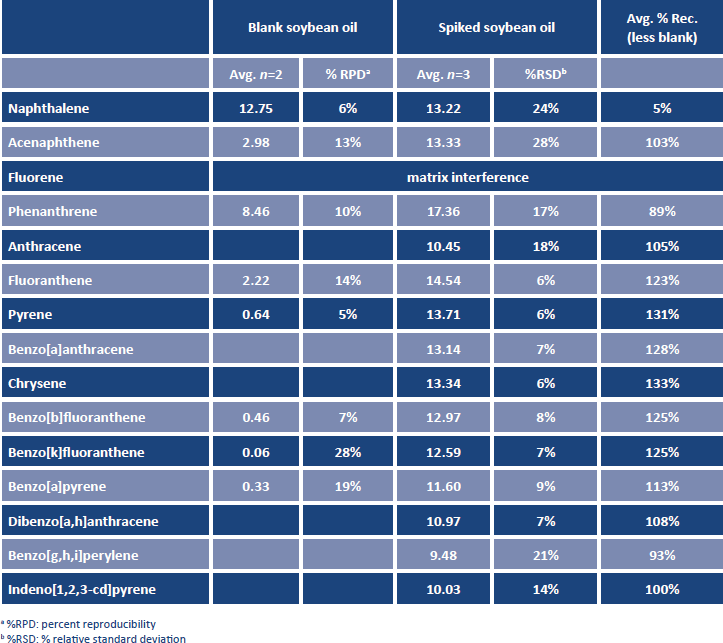
TABLE 4. PAHs recovered from soybean oil, unspiked and spiked at 10 ng/g.
Detectable levels of several PAHs were present in the unspiked oil, and these values were subtracted from amounts determined in the spikes prior to calculation of recovery. Recoveries of >85% were obtained for all PAHs except naphthalene. Low naphthalene recovery was most likely due to evaporative loss during the concentration step. Reproducibility of the spiked replicates, calculated as percent relative standard deviation (%RSD), was <20% for a majority of the analytes. The higher % RSD values for naphthalene and acenaphthene were due to low values obtained for one or more of the spiked samples. This was, again, most likely due to evaporative loss during concentration.
PCBs in fish oil
The same EZ-POP NP procedure used for the soybean oil was repeated with the fish oil; however the resulting extract had very high background. In the spiked samples, only two PCB congeners could be detected free of matrix (Figure 3).
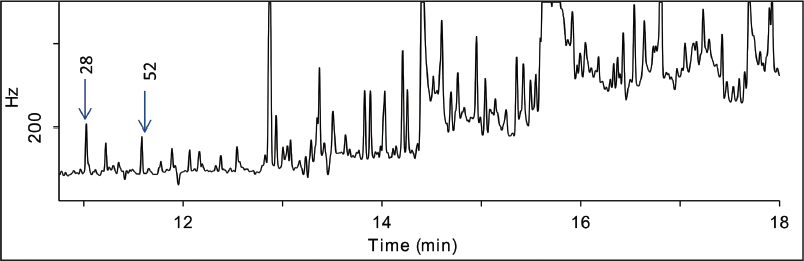
FIG. 3. GC-ECD analysis of spiked fish oil extract prior to secondary silica gel cleanup. Two PCB congeners (indicated) were detected free of background.
For this reason, a secondary cleanup was done with a small silica gel cartridge. This resulted in a very clean background with all congeners detected free of matrix by GC-ECD (Figure 4).
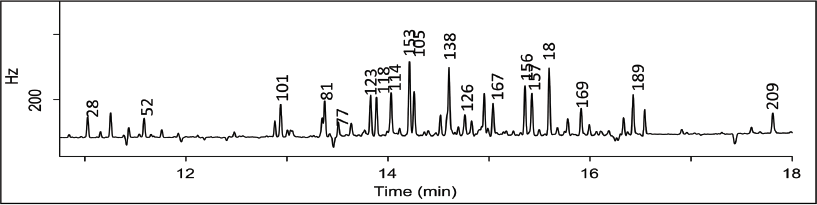
FIG. 4. GC-ECD analysis of spiked fish oil extract (10 ng/g) after secondary silica gel cleanup. PCB congeners are indicated congener number.
Three of the target PCB congeners were detected in the unspiked fish oil (Table 5, page 490), however none of these were coplanar, or "dioxin-like." These blank levels were then subtracted from the spiked samples prior to calculating recoveries. The average recoveries obtained for the set of replicates are summarized in Figure 4. Recoveries were between 75 and 120% for all congeners except no. 209. The recovery of no. 209, or decachorobiphenyl, was lower due to its stronger retention on C18 relative to the other less hydrophobic congeners. Reproducibility, calculated as percent relative standard deviation, is indicated in Figure 4 as error bars. The method yielded good reproducibility, with RSD values < 20% for all analytes, and < 10% for 17 of the 19 congeners.
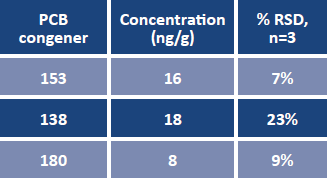
TABLE 5. PCBs detected in unspiked fish oil sample (average n=3).
A new SPE method has been presented for the extraction of nonpolar contaminants from oily samples. The method uses a dual layer SPE cartridge containing Florisil (top bed) and a mixture of C18 and zirconia coated silica, commercially available as "Z-Sep" (bottom bed). This combination of sorbents provides multiple mechanisms for retaining fats; adsorption of polar analytes in the case of Florisil, hydrophobic interaction for the C18, and Lewis acid/base interactions for the Z-Sep. Together, these interactions can retain different constituents of fats such as fatty acids and acylglycerols (mono, di and tri). The EZ-POP NP SPE cartridge has been used in the analysis of PAHs from soybean oil, in a direct method which was easy and quick, and yielded good recoveries and reproducibilities for spiked replicates. For animal-derived fats such as fish oil, a secondary cleanup using a small silica gel SPE cartridge provided an extract clean enough for the GC-ECD analysis of PCB congeners. Recoveries of PCB congeners from spiked cod liver oil were >75% with RSD values of <20% for spiked replicates.
The EZ-POP NP cartridge is a new sample preparation tool for the extraction of nonpolar contaminants from oily and high fat samples. Sample preparation methodologies using EZ-POP NP are simple and effective, and do not require the use of chlorinated or other highly toxic solvents. The resulting extracts can be analyzed by HPLC or GC; and in the case of the later technique, background in many cases should be low enough for analysis on a single quadrupole mass spectral detector. In addition to the applications presented here, EZ-POP NP has been utilized successfully in the GC-MS analysis of PAHs in olive oil, canola oil, and butter; and in the GC-ECD analysis of PCBs in soybean oil (8,9). Future sample preparation work using the EZ-POP NP will investigate its utility in analysis of edible oils and oily food samples for other classes of nonpolar contaminants such as organochlorine pesticides.
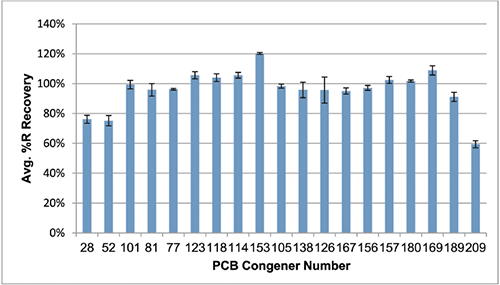
FIG. 5. Average PCB recoveries from spiked fish oil (10 ng/g) after blank subtraction (n=6). The error bars indicate the %RSD for spiked replicates.
Katherine K. Stenerson is a principal scientist at Sigma-Aldrich/Supelco in Bellefonte, Pennsylvania, USA. She can be contacted at Katherine.stenerson@sial.com
Further reading
1. US Environmental Protection Agency International Programs/Persistant Organic Pollutants. http://www.epa.gov/international/toxics.html (accessed Jan. 2013).
2. EU Commission Regulation No 835/2011; Official Journal of the European Union: August 20, 2011 (215) pp 4–8.
3. National Standards of People’s Republic of China, GB 2716-2005: Hygienic Standard for Edible Vegetable Oil. Issued 1/25/2005. Ministry of Health of the People’s Republic of China, Standardization Administration of the People’s Republic of China.
4. European Union Commission. Regulation EC/1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs; Document 2006R1881-EN-01.07.2007-001.001-1; 2006; section 5.10.
5. State of California Environmental Protection Agency Office of Environmental Health Hazard Assessment Safe Drinking Water and Toxic Enforcement Act of 1986. Chemicals Known to the State to Cause Cancer of Reproductive Toxicity, March 27, 2015. http://oehha.ca.gov/prop65/prop65_list/files/P65single03272015.pdf (accessed 4/6/2015).
6. USEPA Superfund Analytical Services, Target Compounds and Analytes. http://www.epa.gov/superfund/programs/clp/som-svtarget.htm#som12 (accessed 4/6/2015).
7. World Health Organization (WHO) Executive Summary, Assessment of the Health Risk of Dioxins: Re-evaluation of the Tolerable Daily Intake (TDI); WHO Consultation: Geneva, Switzerland, May 25–29, 1998.
8. Stenerson, K.K.; Shimelis, O.; Halpenny, M.R.; Espenschied, K.; Ye, M.M. Analysis of Polynuclear Aromatic Hydrocarbons in Olive Oil after Solid-Phase Extraction Using a Dual-Layer Sorbent Cartridge Followed by High-Performance Liquid Chromatography with Fluorescence Detection. J Agric. Food Chem., Article ASAP, DOI: 10.1021/jf506299f, Publication Date (Web): May 4, 2015
9. Stenerson, K.; Brown, C.; Shimelis, O. The Analysis of Persistant Organic Pollutants (POPs) in Oily Samples. Presented at Pittsburgh Conference, New Orleans, LA, March 2015; Paper 2100-5.

![Benzo[a]pyrene Benzo[a]pyrene](/images/informCoverStory/008-01.png)