Science snapshots from Orlando
By Laura Cassiday
July/August 2017
- The 2017 AOCS Annual Meeting and Industry Showcases, held in Orlando, Florida, USA, on April 30–May 3, offered many interesting and informative oral presentations.
- Those who attended could choose from 492 talks given during 68 technical sessions.
- Although complete coverage is not possible, this article highlights several talks that exemplify the high-quality science presented at the meeting.
Orlando, Florida, offers warm temperatures, beautiful scenery, and Mickey Mouse. On April 30 through May 3, 2017, the city also played host to the 2017 AOCS Annual Meeting and Industry Showcases. The 1,389 attendees found the science discussed at the meeting only slightly less thrilling than the rides at Orlando’s famous theme parks. This article provides snapshots of some of the interesting and informative oral presentations given during the meeting.
“Are all fatty acids created equal?”
Presented by David W. L. Ma, University of Guelph, Canada
Health and Nutrition Division
The ratio of n-6 polyunsaturated fatty acids (PUFAs) to n-3 PUFAs in the human diet has risen dramatically over the past century with increasing consumption of vegetable oils. A common perception is that n-6 PUFAs, such as linoleic acid and arachidonic acid, promote inflammation and therefore contribute to conditions such as cardiovascular disease, arthritis, and cancer. On the other hand, n-3 PUFAs, such as α-linolenic acid (ALA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA), are widely considered anti-inflammatory and beneficial to human health. The problem with this simplistic view, says David Ma, is that the structures and biological properties of fatty acids vary widely, even within the n-6 and n-3 families. Moreover, some studies have indicated beneficial effects for n-6 PUFAs in heart health and brain development.
However, studying the biological effects of a particular fatty acid in vivo has been challenging because each fatty acid can be metabolized within the body to other fatty acids with their own distinct effects. The enzyme Δ-6 desaturase catalyzes the first step in the metabolic conversion of both an n-6 PUFA (linoleic acid) and an n-3 PUFA (ALA) into longer-chain fatty acids, such as arachidonic acid, EPA, and DHA. Researchers have generated a transgenic mouse (D6KO) that lacks this enzyme; thus, the mice are unable to metabolize either linoleic acid or ALA (Stroud, C. K., et al., http://doi.org/10.1194/jlr.M900039-JLR200, 2009).
Ma and his colleagues used the D6KO mouse to study whether the pro- or anti-inflammatory effects of linoleic acid and ALA are dependent upon their conversion to other fatty acids. They fed wild-type or D6KO mice one of four diets (rich in linoleic acid, arachidonic acid, ALA, or EPA/DHA) for nine weeks, then sacrificed the mice and examined inflammatory markers within their tissues (Monk, J. M., et al., http://doi.org/10.1016/j.jnutbio.2016.01.004, 2016). The researchers found that the conversion of linoleic acid to arachidonic acid was required for the immune system to activate pro-inflammatory cytokines. In other words, arachidonic acid, but not linoleic acid, was associated with inflammation. On the other hand, ALA showed anti-inflammatory effects independent of its conversion to EPA and DHA.
Ma and his colleagues also used the D6KO mouse model to study the effects of four high-fat diets on the development of fatty liver disease (Monteiro, J., et al., http://doi.org/10.1139/cjpp-2012-0308, 2013). The first diet—the negative control—contained lard (negligible amounts of n-3 and n-6 PUFAs). The other diets contained canola oil (low ALA), flaxseed oil (high ALA), or menhaden oil (positive control; rich in EPA/DHA). The researchers found that the flaxseed-oil-rich diet reduced steatosis (abnormal lipid accumulation) and inflammation in the liver compared with the lard diet, independent of the conversion of ALA to EPA/DHA. However, EPA and DHA had even greater effects in preventing steatosis and inflammation in mice fed a high-fat diet.
During his presentation, Ma also shared some unpublished data from studies examining whether plant- (ALA) or fish- (EPA/DHA) based n-3 PUFAs are more effective for breast cancer prevention in mice. These studies used a transgenic mouse model of breast cancer (MMTV-neu), either by itself or crossed with the D6KO model (MMTV-neu x D6KO). The team found that flaxseed oil reduced tumor size and number in a dose-dependent manner, with a high-ALA diet having similar anti-tumor effects as an EPA/DHA-containing diet.
“The effects of oilseed processing on bioactive compounds in edible canola oil: a case study involving Australian processing plants”
Presented by Clare L. Flakelar, Charles Sturt University, Wagga Wagga, Australia
Processing Division Student Award Winner
Crude canola oil contains 94–98% triacylglycerols, less than 2.5% phospholipids, 0.4–1.2% free fatty acids, and other minor components. These minor components include both undesirable compounds, such as chlorophyll and trace metals, and beneficial ones, such as phytosterols, tocopherols, and carotenoids. A challenge during canola oil processing is to remove the undesirable compounds while retaining the beneficial ones. Graduate student Clare Flakelar wondered whether recent changes in edible oil processing techniques have affected the retention of minor bioactive compounds in canola oil. So she and her colleagues measured the concentrations of phytosterols, tocopherols, and carotenoids in canola oil samples obtained from various stages of processing in five commercial plants in Australia.
These minor components of canola oil are thought to be beneficial to human health. Phytosterols, present at 7,000–10,000 mg/kg of crude canola oil, have been shown to lower LDL cholesterol and may reduce cardiovascular disease risk. Tocopherols, also known as vitamin E, are present at 700–1,200 mg/kg in crude canola oil. As natural antioxidants, tocopherols may reduce the risk of cancer, cardiovascular disease, and other ailments. Finally, carotenoids, such as lutein and β-carotene, occur at levels of 50–150 mg/kg in crude canola oil. Several carotenoids have vitamin A activity and contribute to eye and skin health.
Previous studies have shown that edible oil processing—in particular, the neutralization, bleaching, and deodorization steps—degrades or removes bioactive compounds. But with a recent shift toward milder processing conditions, the retention of bioactive compounds may be enhanced compared with traditional refining methods. To investigate this possibility, Flakelar and her colleagues examined canola oil samples from five Australian commercial plants that use different refining techniques: 1) solvent extraction followed by physical refining (SE-P), 2) expeller press extraction followed by chemical refining (E-C), 3) expeller extraction followed by physical refining (E-P), 4) cold pressing followed by the bleaching stage only (CP), and 5) cold pressing followed by physical refining (CP-P).
The researchers developed a rapid method to simultaneously quantify phytosterols, tocopherols, and carotenoids in canola oil using normal-phase high-performance liquid chromatography (Flakelar, C. L., et al., http://doi.org/10.1016/j.foodchem.2016.07.059, 2017). Using this method, they measured the levels of three phytosterols (β-sitosterol, campesterol, brassicasterol), two tocopherols (α- and γ-tocopherol), and two carotenoids (β-carotene and lutein) in the canola oil samples.
The enrichment of phytosterols in the oil samples fluctuated with each step in the refining process, but the retention of the compounds was high in the finished oils for all processes. The SE-P samples showed higher levels of phytosterols in the finished oil than the other samples. The cold-pressed oils showed the lowest amounts of phytosterols in the finished oils, perhaps because the compounds were not efficiently extracted from the seeds by the cold pressing. The choice of physical or chemical refining did not appear to substantially affect phytosterol content in the expeller-pressed oils (E-P and E-C), which had phytosterol levels in between those of the solvent-extracted and cold-pressed oils.
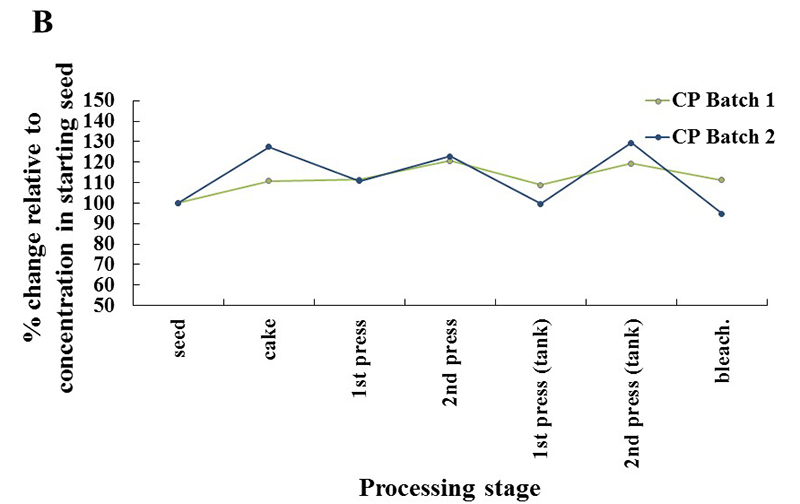
Similar results were obtained for the tocopherols (Fig. 1), with the SE-P oils showing the greatest enrichment in tocopherols relative to the starting seed, and the CP oils the lowest. In all samples, the proportion of α-tocopherol slightly decreased, and the proportion of γ-tocopherol slightly increased, in the finished oils compared with the starting seeds. Under all processing conditions, the level of carotenoids dropped to undetectable after the bleaching step, which was expected because bleaching intentionally removes pigments such as carotenoids. Investigations into new processing methods that retain carotenoids, or extract and purify them as valuable byproducts, are warranted, says Flakelar.
Fig. 1. Changes in tocopherol content with processing stage in canola oil that was (A) solvent extracted and physically refined (SE-P), or (B) cold pressed followed by the bleaching stage only (CP). Credit: Clare Flakelar
This study indicates a high retention of phytosterols and tocopherols in finished oils for all processes, in contrast to previous studies that illustrated dramatic losses during refining. This result could indicate an improved efficiency of refining processes. The study also highlighted differences in the retention of minor components for different pressing techniques. For the CP samples, minor components were increased after a second pressing, but they still remained below the levels of the solvent-extracted samples. These results may guide future efforts to improve the retention of bioactive minor components in edible oils.
“Silicone surfactants in oil-based systems”
Presented by Tony O’Lenick, Siltech, LLC, USA
Surfactants and Detergents Division
JSD 20th Volume Celebration Honoring Milton Rosen
With his book Surfactants and Interfacial Phenomena, now in its fourth edition, Professor Milton Rosen provided a roadmap not only for the characterization and use of conventional surfactants, but also for entirely new classes of surfactant systems. Many of these concepts can be applied to the study of silicone/hydrocarbon surfactants in oil-based systems, says Tony O’Lenick. These non-traditional surfactants can impart a “slippery” feel and silicone-like spread to products ranging from sunscreen to motor oil, and are often more efficient at reducing the surface tension of oils than traditional surfactants.
When mixed together, silicone fluid and oil are immiscible, similar to water and oil. Therefore, surfactants that contain silicone and an alkyl tail should lower the surface tension of oil similar to conventional surfactants that contain a polar head group and a nonpolar tail. Applying Dr. Rosen’s principles, it should be possible to characterize parameters of silicone surfactants such as surface tension lowering, critical micelle concentration (CMC), solubility, emulsification, wetting, and foaming.
Alkyl dimethicones, or silicone waxes, make up one class of silicone surfactants. These molecules consist of an alkyl chain attached to a siloxane backbone. If the length of the alkyl chain is 18 carbons or greater, the silicone wax is solid at room temperature. Silicone waxes with shorter alklyl chains, such as cetyl dimethicone, with 16 carbons in the chain, are liquid at room temperature. Cetyl dimethicone works as a surfactant in soybean oil, lowering the surface tension, improving the spread, and imparting a “silicone-like” feel. Like traditional surfactants, the CMC of cetyl dimethicone can be calculated by plotting the surface tension of the oil versus the weight percent of added surfactant. However, not all silicone surfactants have a readily identifiable CMC. Another measure of effective concentration is the “fixed surface tension.” This value refers to the concentration of silicone surfactant added to reduce the surface tension of the oil to 25 dynes/cm.
Another parameter that can be defined for silicone surfactants is the critical gel concentration (CGC), or the minimum concentration needed to cause a system to gel. C26 dimethicone is a silicone wax with a 26-carbon alkyl chain, making it a solid at room temperature. At its CGC, C26 dimethicone transforms liquid soybean oil into a gel. When added to olive oil at concentrations of 20–80%, C26 dimethicone forms gels of varying firmness and opacity.
Silicone polymer surfactants can act as foaming agents in a triglyceride solution (C8–10). When an air bubble is introduced into the solution, the silicone polymer surfactant reduces the surface tension around the bubble, allowing it to expand rapidly. In addition, silicone polymers entangle with themselves in the liquid border between air bubbles, slowing foam drainage.
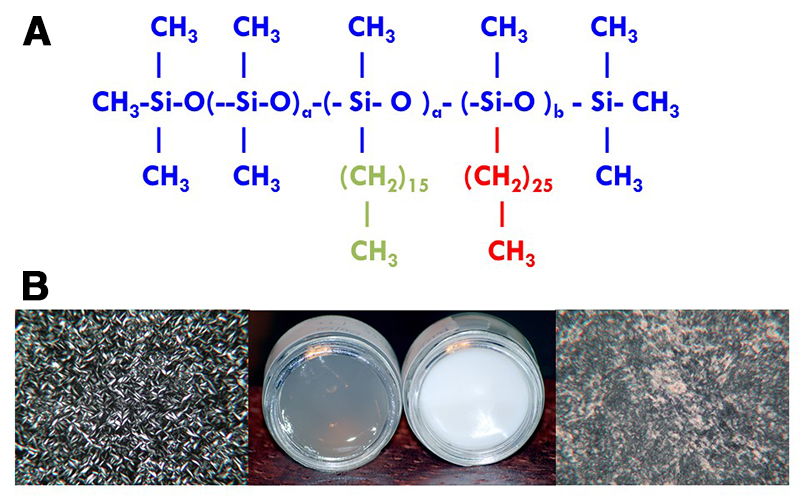
O’Lenick and his colleagues have also studied multi-domain, or gemini, silicone surfactants. Multi-domain alkyl dimethicones contain two different alkyl chains on the same siloxane backbone: a short, liquid carbon chain; and a longer, solid carbon chain (Fig. 2A). These multi-domain silicone surfactants can self-assemble into organized networks that have different properties from mixtures of the corresponding single-domain polymers (Fig. 2B). The multi-domain polymers can form gels that are two-phase liquid crystal systems. These gels have lower melting points, are more translucent, and flow more under pressure compared with a gel made from blending the two single-domain polymers. Thus, the multi-domain silicone surfactants can drastically alter rheology, aesthetics, and other properties of gels.
Fig. 2. A. Chemical structure of a multi-domain alkyl dimethicone surfactant. Shown in green is the short, liquid carbon chain; in red is the longer, solid carbon chain. B. The multi-domain surfactant self-assembles into organized networks (phase-contrast microscope image and gel at left) that are different from those of a polymer blend (right). Credit: Tony O’Lenick
O’Lenick concluded his presentation with the following statement: “Professor Rosen, those of us who were not given the opportunity to be your student; those of us not given the opportunity to listen to your lectures; you have touched our lives and research by your leadership, your roadmap, and most importantly your mentorship. And for that we thank you.”
“Synthetic biology to engineer novel oils with enhanced properties”
Presented by Timothy Durrett, Kansas State University, USA
Biotechnology Division
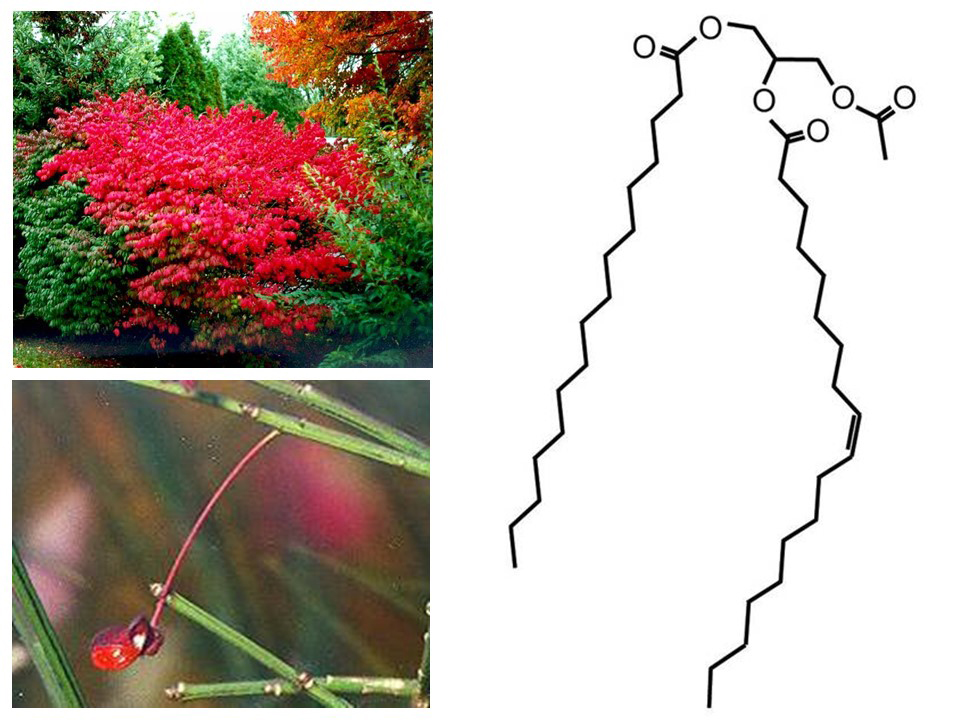
Acetyl-triacylglycerols (acetyl-TAGs) are TAGs that contain an acetyl group in place of the sn-3 fatty acyl group. The unusual structure of acetyl-TAGs confers unique properties that could be useful in biofuels, lubricants, and food emulsifiers. The seeds of the burning bush plant (Euonymus alatus) contain about 50% oil by weight, of which more than 95% is acetyl-TAGs (Fig. 3). By introducing a gene from E. alatus to the oilseed crop Camelina sativa, Timothy Durrett and colleagues produced a transgenic Camelina line that accumulates high levels of acetyl-TAGs in its seeds.
Fig. 3. (Left) The burning bush plant (Euonymus alatus) and seed. (Right) Chemical structure of an acetyl-TAG. Credit: Timothy Durrett
Compared with other vegetable oils, acetyl-TAGs have unique properties. For example, the viscosity of acetyl-TAG oil is about 40% lower than that of soybean oil, which could allow the direct injection of acetyl-TAGs into some types of diesel engine. In contrast, other vegetable oils must be transesterified prior to their use as biodiesel. Acetyl-TAGs also have improved cold-temperature properties compared with regular vegetable oils. Unlike soybean oil, acetyl-TAGs remain liquid at -20 ºC. In addition to possible use as a biofuel, acetyl-TAGs may find applications as lubricants, plasticizers, and food additives (emulsifiers and coatings). Because acetyl-TAGs have an acetyl group in place of a long fatty acid chain, they have about 6.3% fewer calories than regular TAGs.
Using a deep transcript profiling approach, Durrett and his colleagues identified the gene in the burning bush plant that is responsible for the high levels of acetyl-TAGs in the plant’s seeds. The gene encodes an enzyme, Euonymus alatus diacylglycerol acetyltransferase (EaDAcT), that uses acetyl-CoA to acetylate diacylglycerol. Next, the researchers introduced the gene encoding EaDAcT to Camelina sativa, an oilseed crop that requires minimal irrigation and fertilizer, and has a short life cycle. Unlike the burning bush plant, Camelina could cost-effectively produce large quantities of acetyl-TAG oil.
The researchers found that the transgenic Camelina seeds produced oil with about 50% acetyl-TAGs. They were able to further boost acetyl-TAG production in Camelina by suppressing DGAT1, the enzyme responsible for regular TAG synthesis, using RNAi. Seeds that expressed both EaDAcT and DGAT1-RNAi accumulated up to 85% acetyl-TAGs in their oil (Liu, et al., http://doi.org/10.1111/pbi.12325, 2015). Durrett and his colleagues also sequenced the transcriptomes of other acetyl-TAG-producing plants and identified some new enzymes with even higher acetyltransferase activities than EaDAcT. When the researchers introduced a gene encoding one of these enzymes to Camelina, in combination with DGA1-RNAi, the resulting seed oil contained 90% acetyl-TAGs. Importantly, the genetically modified seeds appeared to germinate and grow normally.
To expand the functional repertoire of acetyl-TAG oils, Durrett and his colleagues are now engineering acetyl-TAGs with unusual fatty acids. For example, acetyl-TAGs with medium-chain fatty acids (MCFAs) at the sn-1/sn-2 positions show further reductions in viscosity compared with the long-chain versions, and acetyl-TAGs with ricinoleic acid polymerize differently than their natural acetyl-TAG counterparts. However, the in vivo efficiency of MCFA incorporation into acetyl-TAGs is currently low, and Camelina seeds expressing acetyl-TAGs with ricinoleic acid fail to germinate, indicating that more work is needed in these areas.
“Structured triglycerides in infant formula: development of fat blends with numerous benefits”
Presented by Eric L. Lien, University of Illinois, USA
Health and Nutrition Division
In the first three to four months after birth, an infant’s body weight doubles, fueled by an energy intake that is about four times higher per kilogram per day than that of adults. Breast milk is rich in lipids, and the ability to digest fats is relatively well developed in young infants. Human milk provides superior nutrition for infants, but, some infants cannot be breastfed for various reasons. Therefore, suitable replacements for human milk are needed. In his presentation, Eric Lien summarized what has been learned about the lipid composition of human milk over the past century, how formulators have incorporated this knowledge, and new directions for further improving infant formula so that it more closely mimics breast milk.
Most infant formulas contain the cow’s milk proteins whey and casein, a blend of vegetable oils as a fat source, lactose as a carbohydrate source, vitamins, and minerals. In 1965, British dietitian Elsie Widdowson evaluated a new infant formula that attempted to “humanize” the fat blend (Lancet 2, 1099–1105). The saturated fatty acid profile of the new formula was very similar to that of human milk. Widdowson studied the absorption of fat, protein, and minerals by infants consuming the formula at 5–7 days and 4–6 weeks of age. She found that the formula-fed infants absorbed less fat than the breastfed infants at 5–7 days, but by 4–6 weeks, fat absorption was comparable in the two groups. Surprisingly, calcium retention was drastically reduced (by about 9-fold) in the formula-fed infants compared with the breastfed infants at 5–7 days. The difference in calcium retention was much less pronounced at 4–6 weeks.
In 1968, Tomarelli and colleagues recognized that although the saturated fatty acid profiles of the new formula and human milk were similar, the positioning of palmitic acid (16:0) within triglycerides was quite different, and that this positioning had important metabolic consequences (J. Nutr. 95, 583–590). In human milk, about 70% of the palmitic acid is in the sn-2 position of triglycerides. In contrast, in vegetable oils, which supply the fat blends in infant formulas, most of the palmitic acid is in the sn-1 or sn-3 position. During digestion, pancreatic lipase clips off fatty acids from the sn-1 and sn-3 positions of the triglyceride, releasing two free fatty acids and one sn-2 monoglyceride into the intestinal lumen. Palmitic acid as an sn-2 monoglyceride is much more efficiently absorbed than free palmitic acid, which forms complexes with minerals such as calcium and is excreted in the feces as fatty acid soaps. This finding explained both the reduced fat absorption and the reduced calcium retention observed in formula-fed infants at 5–7 days compared with breastfed infants.
Much later, in 1995, Quinlan et al. examined fatty acid soaps in the stools of breastfed and formula-fed infants (J. Pediatr. Gastroenterol. Nutr. 20, 81–90). The researchers found that soap fatty acids comprised about 6% of the wet weight of stools from formula-fed infants, compared with only about 0.5% of stools from breastfed infants. In addition, the formula-fed infants excreted about four times more calcium in their stools than breastfed infants. The formula-fed infants also had harder stools than the breastfed infants.
In the late 1990s, structured triglycerides with palmitic acid at the sn-2 position were synthesized from vegetable oils using an enzymatic process. About 50% of the palmitic acid in these triglycerides is in the sn-2 position. In 1999, Kennedy et al. assessed the new lipid blend by comparing three groups of healthy newborn infants: control formula-fed, high-sn-2 formula-fed, and breastfed (Am. J. Clin. Nutr. 70, 920–927). After 12 weeks of feeding, stool characteristics and bone mineral density (as an indicator of calcium retention) were determined. The researchers found that the level of palmitic acid soaps in the stools of the high-sn-2 formula-fed infants was in between that of the control formula-fed and breastfed infants. The bone mineral density and stool consistency of the high-sn-2 formula-fed infants also more closely resembled those of the breastfed infants.
In the final part of his talk, Lien discussed some new approaches to improving the composition of infant formula, such as adding the prebiotic oligofructose to high-sn-2 formula. Oligofructose serves as a readily digestible carbohydrate source for some potentially beneficial colonic bacteria, such as bifidobacteria. These beneficial bacteria tend to be higher in the colon and feces of infants fed breast milk than those fed standard (non-high-sn-2) formula. In 2014, Lien and his coworkers compared the stool consistency and composition of healthy newborn infants fed either control formula, high-sn-2 formula with or without oligofructose, or human milk (Yao, et al., http://doi.org/10.1097/MPG.0000000000000443).
The researchers found that the addition of oligofructose to the high-sn-2 formula led to softer stools in the infants, but the amounts of fatty acid soaps and calcium in the infants’ stools were not significantly different from the group consuming high-sn-2 formula without oligofructose. Unexpectedly, the researchers uncovered an apparent prebiotic effect of the sn-2 formula itself: Infants fed high-sn-2 formula with or without oligofructose showed comparable levels of stool bifidobacteria as those fed breast milk. In contrast, the control formula-fed infants had significantly lower levels of bifidobacteria in their stools. The growth of bifidobacteria may be promoted by sn-2-palmitate, or inhibited by palmitate-calcium soaps, the researchers suggest.
Laura Cassiday is an associate editor of INFORM at AOCS. She can be contacted at laura.cassiday@aocs.org.
INFORMation
- Flakelar, C. L., et al. (2017) “A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography.” Food Chem. 214, 147–155. http://doi.org/10.1016/j.foodchem.2016.07.059
- Kennedy, K., et al. (1999) “Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization.” Am. J. Clin. Nutr. 70, 920–927.
- Liu, J., et al. (2015) “Metabolic engineering of oilseed crops to produce high levels of novel acetyl glyceride oils with reduced viscosity, freezing point and calorific value.” Plant Biotechnol. J. 13, 858–865. http://doi.org/10.1111/pbi.12325
- Monk, J. M., et al. (2016) “The delta 6 desaturase knock out mouse reveals that immunomodulatory effects of essential n-6 and n-3 polyunsaturated fatty acids are both independent of and dependent upon conversion.” J. Nutr. Biochem. 32, 29–38. http://doi.org/10.1016/j.jnutbio.2016.01.004
- Monteiro, J., et al. (2013) “Oils rich in α-linolenic acid independently protect against characteristics of fatty liver disease in the Δ-6 desaturase null mouse.” Can. J. Physiol. Pharmacol. 91, 469–479. http://doi.org/10.1139/cjpp-2012-0308
- Quinlan, P. T., et al. (1995) “The relationship between stool hardness and stool composition in breast- and formula-fed infants.” J. Pediatr. Gastroenterol. Nutr. 20, 81–90.
- Stroud, C. K., et al. (2009) “Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration.” J. Lipid Res. 50, 1870–1880. http://doi.org/10.1194/jlr.M900039-JLR200
- Tomarelli, R. M., et al. (1968) “Effect of positional distribution on the absorption of the fatty acids of human milk and infant formulas.” J. Nutr. 95, 583–590.
- Widdowson, E. M. (1965) “Absorption and excretion of fat, nitrogen, and minerals form “filled” milks by babies one week old.” Lancet 2, 1099–1105.
- Yao, M., et al. (2014) “Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity.” J. Pediatr. Gastroenterol. Nutr. 59, 440–448. http://doi.org/10.1097/MPG.0000000000000443
You can make next year’s meeting even better
Planning for next year’s annual meeting begins almost as soon as this year’s meeting is over. So, now is the time to start thinking about how you can best use your unique knowledge, experience, and skills to make the 2018 AOCS Annual Meeting & Expo in Minneapolis, Minnesota, USA, May 6¬–9, a little better than this year’s meeting. Perhaps you have an idea for a session topic that didn’t get covered this year, or could add a fresh perspective by chairing a session next year. You could present your research, organize a symposium, recognize a colleague, or even become a Division leader. There are so many opportunities to take part in this ultimate collaboration of industry, academia, and government. Discover the possibilities at AnnualMeeting.aocs.org/2018.