Pulses rising
By Laura Cassiday
April 2018
- Pulses are legumes harvested solely for their dry seeds, such as chickpeas, lentils, peas, and beans.
- Manufacturers are incorporating pulses, a good source of plant-based protein and other nutrients, into a wide array of foods.
- Challenges facing the pulse industry include improving protein quality, reducing antinutritive factors, enhancing sensory attributes, and accelerating pulse breeding and agricultural innovation.
Pulses such as chickpeas, lentils, peas, and beans are culinary staples in many parts of the world. Stews, hummus, falafel, chilies, and curries all feature the flavorful and nutritious dried legumes. However, these days pulses are showing up in unexpected places—in pastas, breads, snack foods, beverages, and meat and dairy substitutes. In addition to being rich in protein and fiber, pulses are widely considered more sustainable than animal-based proteins. To heighten awareness of the benefits of pulses, the United Nations Food and Agriculture Organization (FAO) declared 2016 the “International Year of Pulses.”
Members of the legume family, pulse seeds grow in pods and vary widely in shape, size, and color. The FAO defines pulses as crops that are harvested solely for their dry seeds. This definition excludes oilseeds, such as soybean and peanut, as well as seeds that are eaten in their immature form as vegetables, such as green peas and green beans. The FAO has recognized 11 primary pulses, including dry beans (e.g., kidney, lima, pinto, navy), dry broad beans (fava, horse), dry peas (garden), chickpeas, dry cowpeas (black-eyed pea), pigeon peas, lentils, bambara beans (groundnuts), vetches (used mainly for animal feed), lupines (used mainly for animal feed and as ornamental flowers), and minor pulses (winged bean, guar bean) (https://tinyurl.com/FAO-pulses).
Changing diets
Archeological sites in India, Egypt, Mesopotamia, the Mediterranean, and Switzerland indicate that humans have been cultivating pulses for thousands of years. Currently, India leads the world in the production and consumption of pulses. Other major producers, in order of total production, include Canada, Myanmar, China, Brazil, and Australia (FAO, https://tinyurl.com/FAO-pulsebook, 2016). From 1990 to 2014, pulse production grew at the fastest annual rate (7.7%) in North America, largely due to a greater than 10-fold increase in Canadian pulse production.
Although worldwide pulse production is increasing, per capita consumption has witnessed a long-term decline. From 1961 to 2001, India’s per capita pulse consumption decreased from 24 kg to 12 kg per year (Canadian Special Crops Association, https://tinyurl.com/CSPA-2014, 2014). During the same period, China’s pulse consumption fell from 10 kg to 1 kg. Meanwhile, North America’s per capita pulse consumption remained modest but steady at about 3.5 kg per year. In developing economies such as India and China, increased urbanization and rising incomes have contributed to reductions in pulse consumption, with concomitant increases in the consumption of meat, dairy, and processed foods.
However, as the world population soars toward an estimated 9.6 billion in 2050, experts predict that meat will become scarce and expensive, and the demand for plant-based proteins like pulses will rise dramatically. Projections indicate a 23% increase in global pulse consumption by 2030, with a more rapid increase (about 50%) in Africa (Global Pulse Confederation, https://tinyurl.com/GPC-strategy, 2016).
Investing in pulses
Many governments, universities, food companies, and investors have recognized the potential of pulses. Canada is emerging as a pulse powerhouse. In the early 1900s, research conducted by the Canadian Department of Agriculture (now known as Agriculture and Agri-Foods Canada; AAFC) identified two varieties of field peas that are suitable for growth in Canada. “Further research carried out since the late 1970s by AAFC and Canadian universities has developed suitable varieties of beans, lentil, chickpea, faba bean, and other pulses for prairie climate and growing seasons, while providing high yield and resistance to pests and pathogens,” says Janitha Wanasundara, research scientist at AAFC, in Saskatoon, Saskatchewan. The fruits of these labors are being realized: Canada is now the world’s largest pulse exporter, with most exports going to India (https://tinyurl.com/FAO-pulsebook).
Canadian pulse production, which includes beans, chickpeas, lentils, and peas, takes place primarily in the provinces of Saskatchewan, Alberta, and Manitoba. Canada leads the world in the production of dry peas, which are attracting increasing interest from food companies as a source of pea flour and protein. In September 2017, French food company Roquette announced that it would build the world’s largest pea-processing plant in Portage la Prairie, Manitoba. Also in 2017, Verdient Foods launched a “massive” organic pea-processing plant (funded in part by movie director James Cameron) in Vanscoy, Saskatchewan (Hui, A., https://tinyurl.com/pulses-future, 2017).
However, the pulse boom in Canada is showing signs of a slowdown in 2018 (Skerritt, J., https://tinyurl.com/pulse-bust, 2018). A surge in pea and lentil production since 2014 led to a global oversupply, which caused prices to plummet. In December 2017, India imposed a 30% duty tax on chickpea and lentil imports. Now farmers have swapped some of their pulse acreage for canola and wheat. According to the AAFC, pea plantings in Canada will likely decline to a seven-year low in the spring of 2018, whereas lentil acreage will drop 27%.
US-based company Cargill (Minnetonka, Minnesota) is investing in pea-based ingredients. In January 2018, the company signed a joint venture agreement with PURIS (Minneapolis, Minnesota), North America’s largest producer of pea protein (Wyers, R., https://tinyurl.com/pea-protein-surge). PURIS produces pea protein, starch, fiber, and flour from organic, non-genetically modified (non-GM) peas grown in the Midwestern United States. Cargill’s financial backing will allow PURIS to expand its operations and build a second plant in the United States.
Pulse appeal
Consumers are increasingly attracted to pulses because they satisfy several trends: plant-based protein, non-GMO, gluten-free, and clean label. Increased globalization has made people more aware of pulses, says Wanasundara. “The food items on our plate have been changing,” she says. “Migration of different cultural groups across the globe, increasing awareness of different cuisines, and the search for new ingredients have contributed to an increased use of pulses.”
“In Europe, people are becoming more aware of the problems caused by the massive production of animal-based foods, so there’s an interest in plant proteins,” says Raffael Osen, deputy head of the Department of Process Development at the Fraunhofer Institute for Process Engineering and Packaging in Freising, Germany. “Many consumers have problems with soy due to GMO issues, as well as the image that soy is grown in former rainforest areas in South America.” In contrast, consumers are familiar with peas, beans, and lentils, which are “locally grown” in European countries.
Because ingredients like “pea protein” and “lentil flour” are easily understood by most consumers, manufacturers can use them to replace synthetic or less familiar ingredients for clean-label claims. And since pulses are gluten-free, they appeal to consumers with celiac disease, as well as the increasing number of people who consider themselves sensitive to gluten. Some food manufacturers who incorporate pulse-based ingredients advertise their products as “allergen-free” or “allergen-friendly” to differentiate them from soy protein, which must be labeled as an allergen in North America, the European Union, and other areas.
“I think this form of product positioning is a bit premature,” says Phil Kerr, president and founder of SERIO Nutrition Solutions, LLC, in St. Louis, Missouri, USA. “In many instances, the reason why foods are on the major allergen list is because there’s large exposure to the global population. As the exposure to pulses gets larger, they may very well be recognized as allergens like other legumes, such as peanuts and soybeans.” Indeed, allergies to lentils and lupines, which often overlap with peanut allergies, have already been reported. In addition, about 400 million people worldwide have a genetic disorder called glucose-6-phosphate dehydrogenase deficiency, which makes them susceptible to “favism”—a hemolytic response to fava bean consumption.
Protein quantity and quality
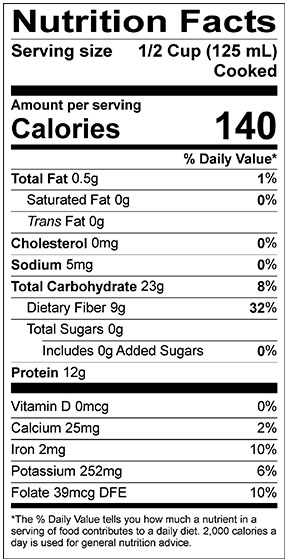
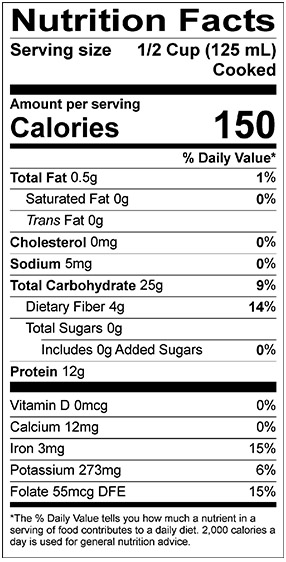
With a crude protein content of approximately 21–26% by weight, pulses are a much better source of protein than cereal grains such as wheat, barley, and quinoa (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). The protein content of pulses can be influenced by both genetic and environmental factors. For example, the protein content in 59 different pea lines ranged from 13.7–30.7% by weight, with an average of 22.3% (Tzitzikas, E.,N. et al., http://dx.doi.org/10.1021/jf0519008, 2006). Pulses are also rich in complex carbohydrates and dietary fiber, low in fat, and a good source of several vitamins and minerals such as calcium, folate, potassium, and iron (Fig. 1).
FIG. 1: Nutrition facts for A) whole green lentil (boiled) and B) split red lentil (boiled). Credit: Lentils.org
To increase the protein content of pulse ingredients, manufacturers make pulse protein concentrates or isolates (Nosworthy, M.G., et al., http://dx.doi.org/10.1094/CFW-62-4-0139, 2017). Pulse protein concentrates are produced by a “dry” method called air classification. Seeds are milled to produce a fine flour. Then, the flour is passed through a spiral air stream to separate fine particles (protein) from coarse particles (starch). Air classification does not completely remove starch granules from the protein fraction, but it does increase the overall protein content. For example, in one study, air classification increased the protein content of peas from 21.5 to 56.3%, of lentils from 19.5% to 49.3%, and of fava beans from 31.6 to 75.1% (Elkowicz, K, and Sosulski, F.W., https://doi.org/10.1111/j.1365-2621.1982.tb07673.x, 1982).
In contrast, pulse protein isolates are produced by the “wet” method of alkaline extraction followed by protein precipitation (Nosworthy, M.G., et al., http://dx.doi.org/10.1094/CFW-62-4-0139, 2017). Milled flour is incubated in an alkaline solution (pH 8–11), which solubilizes most of the protein but not other flour components, such as starch. Insoluble material is removed by filtration or centrifugation. Then, the pH of the solution is decreased to an appropriate isoelectric point, and the protein precipitates. The protein precipitate is collected by centrifugation and washed, neutralized, and dried. Protein contents of 90.1–90.8%, 86.7–89.3%, and 95.3% for pea, lentil, and fava bean isolates, respectively, have been reported (Nosworthy, M.G., et al., http://dx.doi.org/10.1094/CFW-62-4-0139, 2017).
Isolates have a higher protein content than concentrates. In addition, the alkaline extraction reduces the activity of antinutritive factors that affect protein digestibility, such as trypsin inhibitors and hemagglutinins. As a result, pulse protein isolates are generally more digestible than concentrates.
In the European Union, food manufacturers can make protein claims on packages based solely on protein content, and not quality (Marinangeli, C.P.F., and House, J.D., https://doi.org/10.1093/nutrit/nux025, 2017). If a food derives 12% of its energy from protein, it can be labeled as a “source of protein.” Greater than 20% energy from protein qualifies for a “high source or protein” claim. However, in North America, claims of “good” or “excellent” sources of protein require evidence of protein quality (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). The quality of a dietary protein is determined by two factors: 1) the amino acid composition and its correspondence with human amino acid requirements, and 2) the digestibility of the protein. Typically, proteins from animal sources (beef, chicken, pork, eggs, and dairy) are the best suited to human requirements, while also being highly digestible. In contrast, plant-based proteins often lack one or more indispensable amino acids, and they are less digestible than animal proteins.
There are three major methods to determine protein quality, each with its own advantages and disadvantages (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017) (Fig. 2). The Protein Efficiency Ratio (PER), which is the method accepted by the Canadian government, uses a rodent feeding trial to determine the quality of a test protein compared with a reference protein (the dairy protein casein). Researchers feed an experimental diet or a casein control diet, which each consists of 10% protein, to weanling rats for 28 days. Then, the total weight gain of the rats is divided by the amount of protein consumed. To standardize PER across laboratories, the raw PER value is adjusted to the average PER of casein (2.5). The protein rating of a food is calculated by multiplying the adjusted PER by the grams of protein in a reasonable daily intake. For example, white bread has 12.6 g of protein in a reasonable daily intake of 150 g (8.4% protein), and a PER of 1.0, so the protein rating is 12.6. In Canada, foods must have a protein rating of at least 20 to be labeled a “good” source of protein, and of at least 40 to be considered an “excellent” source of protein.
FIG. 2: Three methods for calculating protein quality for protein quality claims: PER (A), PDCAAS (B), and DIAAS (C). PER, used by Canada, measures weight gain in rats over 28 days as a function of their protein intake. PDCAAS, used by the United States, takes into account the amino acid score of a protein and its true fecal nitrogen digestibility. DIAAS, recommended by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) but not yet implemented by any country, considers the amino acid score of a protein and the ileal digestibility of each indispensable amino acid (IAA). Credit: Adapted from Marinangeli, C.P.F., and House, J.D., http://dx.doi.org/10.10.93/nutrit/nux025, 2017.
PER is a simple calculation that requires only the measurements of weight gain and protein intake. Another advantage is that PER reveals the actual impact of a protein source on growth. However, the method assumes that all of the protein the rat consumes is used for growth, and neglects contributions to maintenance and other metabolic processes. Also, rats require much higher levels of the sulfur-containing amino acids (cysteine and methionine) than humans do, which could underestimate the quality of many pulse proteins that are limiting in these amino acids (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017).
In 1989, the FAO and the World Health Organization (WHO) established the Protein Digestibility Corrected Amino Acid Score (PDCAAS) as the preferred method to measure protein quality (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). PDCAAS is a more quantitative measure of protein quality than PER: the method measures the amount of each indispensable amino acid in a protein sample, as well as the protein sample’s digestibility. The amino acid score reflects the amount of each indispensable amino acid (lysine, methionine plus cysteine, threonine, and tryptophan) in a protein sample compared with the amount of the same amino acid in a reference provided by the FAO (typically, the essential amino acid requirements of a child aged 6 months to 3 years). An amino acid score of 1.0 or greater indicates no deficiency in that amino acid. The amino acid with the lowest score is considered to be the first limiting amino acid in that protein, and its amino acid score is used in the calculation of PDCAAS. In the United States, PDCAAS is used to regulate most protein claims.
The other portion of the PDCAAS calculation, true fecal nitrogen digestibility, is determined by a rodent assay (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). Rats are fed a controlled diet, and the amount of nitrogen in their feces is subtracted from the amount of nitrogen they ingested, with a correction for the contribution of endogenous protein to the total fecal nitrogen. To calculate PDCAAS, the lowest amino acid score is multiplied by the true fecal nitrogen digestibility. PDCAAS values are truncated at 1.0 (the value set for casein). Then, the corrected protein level can be determined by multiplying the PDCAAS by the amount of total protein in a serving. Finally, the percent daily value is calculated by dividing the corrected protein level by 50 g, the daily reference value for protein recommended by the US Food and Drug Administration. For a product to make a “good” source of protein claim, it must have a percent daily value of at least 10%. An “excellent” source of protein contains at least 20% of the daily reference value.
In 2011, an Expert Consultation of the FAO and WHO recommended replacing the PDCAAS with a different measure of protein quality, the Digestible Indispensable Amino Acid Score (DIAAS) (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). The DIAAS is thought to be more accurate than the PDCAAS because it measures ileal digestibility, rather than fecal digestibility. Feces typically contain a significant amount of bacterial protein contaminants, with a final amino acid composition that is quite different from that in the ileum (the final section of the small intestine). For example, in growing pigs, fecal digestibility is about 22% greater than ileal digestibility, which could cause protein quality to be overestimated by PDCAAS. Another advantage is that DIAAS considers the ileal digestibility of each amino acid in a protein sample, rather than the digestibility of the total protein, as in PDCAAS.
To calculate DIAAS, researchers compare the amount of each digestible indispensable amino acid in a protein sample with that of a reference pattern, and multiply this ratio by 100. The DIAAS corresponds to the lowest amino acid score for the indispensable amino acids. Unlike PDCAAS, DIAAS values are not truncated, making it easier to assess the quality of protein blends. For DIAAS, protein claims are based on both quantity and quality. Foods with a crude protein content of 5–9.9 g and DIAAS values of at least 75 qualify for a “good source of protein” claim, whereas foods with a crude protein content of at least 10 g and a DIAAS of at least 100 are “excellent” sources of protein.
Although DIAAS likely provides a more accurate assessment of protein quality than PER or PDCAAS, ileal sampling is quite invasive. Therefore, most of the data on ileal digestibility comes from pig or rodent models, rather than humans (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). Because of this and other scientific and economic hurdles, a 2014 FAO/WHO Expert Consultation recommended that additional research be conducted before DIAAS replaces PDCAAS as the standard method to validate protein claims (Lee, W.T.K., et al., http://doi.org/10.3945/jn.115.222109, 2016). As of yet, no jurisdiction uses the DIAAS to verify protein claims.
PDCAAS values are usually higher than DIAAS values for the same protein, but the differences are generally small (1.8–9%) for plant-based proteins (Marinangeli, C.P.F., and House, J.D., https://doi.org/10.1093/nutrit/nux025, 2017). However, because the cutoff for DIAAS protein claims is set relatively higher than for PDCAAS claims, some pulse proteins that would qualify as “good” sources of protein under the PDCAAS system would be ineligible for a protein claim under the DIAAS method.
Pulses vary in their limiting indispensable amino acid. Cooked Canadian red kidney beans, whole green lentils, split red lentils, split green peas, and black beans are limited by the sulfur-containing amino acids (methionine and cysteine) (Nosworthy, M.G., et al., http://dx.doi.org/10.1002/fsn3.473, 2017). In contrast, cooked Canadian navy beans, split yellow peas, chickpeas, and pinto beans are limited by tryptophan.
Table 1 shows the amino acid score, total protein digestibility, and PDCAAS of several pulses, as well as wheat flour, rice flour, soy, and casein. Pulses generally have a higher PDCAAS than cereal grains, but a lower PDCAAS than soy or animal proteins (e.g., casein). Although pea protein isolates are more digestible than concentrates, they have a lower amino acid score because the alkaline extraction process alters the amino acid composition of the isolated protein. Therefore, the PDCAAS values of pea protein isolate and pea protein concentrate are roughly equivalent (Table 1).
| TABLE 1: PDCAAS values for various protein sources | |||
| Protein | Amino Acid Score | True Protein Digestibility (%)* | PDCAAS |
| Pea (yellow, split) | 0.73 | 87.9 | 0.64 |
| Pea (green, split). | 0.59 | 85.2 | 0.50 |
| Lentil (green, whole) | 0.71 | 87.9 | 0.63 |
| Lentil (red, split) | 0.59 | 90.6 | 0.54 |
| Chickpeas (Kabuli) | 0.61 | 85.0 | 0.52 |
| Pinto Beans | 0.77 | 76.2 | 0.59 |
| Kidney Beans | 0.70 | 78.6 | 0.55 |
| Black Beans | 0.76 | 70.0 | 0.67 |
| Navy Beans | 0.83 | 80.0 | 0.67 |
| Wheat Flour | 0.47 | 92.3 | 0.43 |
| Rice Flour | 0.54 | 92.0 | 0.50 |
| Soy Flour (50% protein) | 0.92 | 83.5 | 0.77 |
| Pea protein Isolate (82% protein) | 0.54 | 97.1 | 0.53 |
| Pea Protein Concentrate (50% protein) | 0.58 | 92.6 | 0.54 |
| Soy Protein Isolate (93% protein) | 0.87** | 96.0 | 0.84 |
| Casein | 1.04 | 96.6 | 1.00 |
| *True fecal nitrogen digestibility **Other sources (e.g., Hughes, G.J., et al., http://dx.doi.org/10.1021/jf203220v, 2011) have calculated a PDCAAS for soy protein isolate of 1.00. Credit: "Protein quality of cooked pulses," Pulse Canada |
|||
Many pulse proteins do not have sufficiently high PDCAAS values to make protein claims at the amount of protein present in a typical serving. However, combining pulse proteins with other plant protein sources, such as cereal grains, can improve the overall amino acid score, and thus the weighted-average PDCAAS. Pulses tend to be higher in lysine and lower in sulfur-containing amino acids than cereal grains such as wheat and rice, whereas cereals are lower in lysine and higher in the sulfur-containing amino acids. “We can partner pulses with other commodities that offset their amino acid deficiencies,” says James House, professor and head of the Department of Food and Human Nutritional Sciences at the University of Manitoba, in Winnipeg, Canada. “The classic example is the South American staple of beans and rice. By creating pulse-cereal blends, we can obtain higher-quality proteins.”
Possible health benefits of pulses
In addition to being a good source of protein, pulses may benefit cardiovascular health, weight management, and gastrointestinal function (Dahl, W.J., et al., https://doi.org/10.1017/S0007114512000852, 2012). Some studies also suggest that pulses could act as prebiotics by improving intestinal microbial homeostasis. However, evidence for these benefits is currently inconsistent. Long-term studies of large populations are required to investigate possible roles of pulses in disease prevention. Pulses are high in resistant starch, which is less easily broken down into glucose by digestive enzymes in the small intestine and then absorbed into the blood than other forms of starch. Bacteria in the large intestine transform resistant starch into short-chain fatty acids, which contribute to gastrointestinal health. The high contents of resistant starch and fiber in pulses make them low-glycemic foods, which could be beneficial in the prevention and management of type 2 diabetes (Dahl, W.J., et al., https://doi.org/10.1017/S0007114512000852, 2012).
With a glycemic index (GI) of 39–55, pulses are less likely to affect blood sugar levels than white rice (GI 80), white bread (GI 100), and potatoes (GI 121). Studies suggest that including pulse flours in cereal-based food products can help reduce the glycemic profile of these foods. For example, an extruded breakfast cereal containing 56.5% pea flour or pea semolina, 31.5% corn meal, 6.5% pea fiber, 0.5% salt, and 5% sugar released about 20% less glucose in vitro than the same cereal lacking the pea ingredients (Pulse Canada, https://tinyurl.com/pulsecanada-pea).
In a crossover clinical trial involving 23 overweight, hypercholesterolemic men and women, researchers compared the effect of muffins made with white-wheat flour, whole pea flour, or fractionated pea flour (pea hulls only) on insulin resistance over a 28-day period (Marinangeli, C.P, and Jones, P.J., https://doi.org/10.1017/S0007114510003156, 2011). They found that eating two muffins a day containing either whole pea flour or fractionated pea flour reduced fasting insulin levels by about 20% compared with muffins containing wheat flour. Also, the muffins containing pea ingredients lowered estimates of insulin resistance by about 25% compared with the muffins made from wheat flour.
A recent meta-analysis of 21 clinical trials involving 940 adults found that eating one serving per day of beans, peas, chickpeas, or lentils could contribute to modest weight loss (Kim, S. J., et al., https://doi.org/10.3945/ajcn.115.124677, 2016). The study participants lost an average of 0.34 kg (0.75 pounds) over 6 weeks by incorporating one serving a day of pulses, without making an effort to reduce other foods. The weight loss may have been due to the low GI of pulses and/or the increased feeling of satiety that pulses appear to induce. “Though the weight loss was small, our findings suggest that simply including pulses in your diet may help you lose weight, and we think more importantly, prevent you from gaining it back after you lose it,” says Russell de Souza, assistant professor at McMaster University, in Hamilton, Canada.
Antinutritive factors
Although rich in several nutrients, pulses also contain antinutritive factors that limit digestibility and nutrient absorption, including phytic acid, protease inhibitors, phenolic compounds (tannins, phenolic acids), lectins (hemagglutinins), saponins, and oxalates. Phytic acid is a storage form of phosphorous that binds minerals and prevents their absorption by the small intestine. A good source of some minerals, such as potassium, phosphorus, magnesium, and calcium, pulses could help correct micronutrient deficiencies. However, phytic acid limits the bioavailability of the minerals in pulses, particularly calcium, zinc, and iron. Trypsin and other proteases are enzymes involved in the digestion of dietary proteins. Protease inhibitors interfere with their actions, thus reducing the digestibility of pulse proteins. Similarly, tannins form complexes with proteins and digestive enzymes, inhibiting their activities.
Cooking pulses can alter the amount of antinutritive factors (Nosworthy, M.G., and House, J.D., http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI, 2017). The method of cooking appears to affect antinutrient composition. For example, traditional cooking methods (roasting, boiling) are more effective at destroying lectins, whereas microwaving is better at reducing trypsin inhibitor activity. Food processing techniques such as soaking, autoclaving, micronization, extrusion, germination, and fermentation also alter the antinutritive content and, therefore, protein digestibility and nutrient availability.
Pulse sustainability
Many consumers view pulses as sustainable, environmentally friendly alternatives to animal protein sources. Pulses require less water, energy, and nitrogen fertilizer to produce than most animal and plant protein sources (Global Pulse Confederation, https://tinyurl.com/GPC-strategy, 2016). Many pulse crops are well adapted to arid and semi-arid climates, and they can tolerate drought and frost stress better than most crops. Pulse cultivation occurs over a wide geographical area. “In contrast to soy, where three countries—the Unites States, Brazil, and Argentina—really dominate the global supply, pulse crops are grown all over the world,” says Kerr.
For many years, farmers have recognized the key role that pulses and other legumes play in crop rotation. Legumes harbor symbiotic, nitrogen-fixing bacteria in structures called root nodules. These bacteria, known as rhizobia, fix nitrogen gas (N2) from the atmosphere into ammonia (NH3), which is eventually released from the plant’s roots into the soil. In this way, pulses are a more environmentally friendly alternative to nitrogen fertilizers.
Food applications
Pulse flours, concentrates, isolates, and extracts are being incorporated into a wide range of foods beyond the traditional uses, including breads, pastas, snacks, breakfast cereals, meats, desserts, and beverages. Pulse flour has been used to wholly or partially replace wheat flour in baked goods and pasta. Chickpea brine can be whipped into a foam and used as an egg white substitute. Pulse purees have been used in desserts to reduce the fat and butter content. Pea protein is being used to make dairy-free cheeses. Pea starch can replace cornstarch in coatings for French fries, mozzarella sticks, and onion rings. Pulse ingredients are being explored as emulsifiers and foaming, gelling, and thickening agents in sauces and beverages. Beyond Meat, a company based in Los Angeles, California, USA, sells plant-based burgers made from pea protein isolates and chicken substitutes made from a mixture of pea and soy proteins.
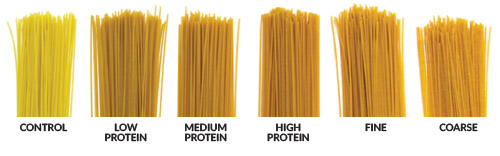
In one study, yellow pea flour was incorporated into a spaghetti formulation at 30% inclusion with durum semolina (Pulse Canada, https://tinyurl.com/Pulsecanada-pasta). The resulting spaghetti had similar quality attributes to 100% durum spaghetti, including texture, cooking time, and cooking loss. The spaghetti containing pea flour was also higher in protein and fiber than the 100% durum spaghetti. Color was the major difference between the two pastas. The spaghetti made with pea flour was less bright, more red, and firmer than the regular spaghetti (Fig. 3A). However, by adjusting the drying conditions of the pea-flour-containing spaghetti, researchers could increase the brightness of the pasta by 20%, reduce the redness by more than 50%, and decrease the firmness by 30% (Fig. 3B).
FIG. 3: (A) Spaghetti made with 100% durum semolina (control) or 30% yellow pea flour/70% durum semolina with different protein contents and fine or coarse flour milling.(B) Cooked spaghetti made with 30/70% yellow pea flour/durum semolina, prepared under high temperature-short time drying conditions (top row) or low temperature-long time drying conditions (bottom row). Credit: Pulse Canada, https://tinyurl.com/Pulsecanada-pasta
Pulses flours have also been tested as binders, fillers, and extenders in ground meat products. For example, Wanasundara, Phyllis Shand at the University of Saskatchewan, and colleagues tested chickpea flour as an extender (a non-meat substance with substantial protein) for low-fat pork bologna (Sanjeewa, W.G.T., et al., https://doi.org/10.1016/j.foodres.2009.07.024, 2010). To reduce the fat content of processed meat, formulators can substitute water for fat, but this typically changes the texture and water-holding ability of the meat. However, adding 5% chickpea flour to low-fat bologna increased the firmness, decreased water release, and increased cook yield, with flavor properties similar to the control bologna.
In another study, Wanasundara and colleagues with InfraReady Products (Saskatoon, Canada) found that adding micronized lentil flour to red meat improves color stability and slows lipid oxidation in fresh and frozen meat (Cross, B., https://tinyurl.com/lentils-beef, 2013). Micronization is a process in which a substance is treated with infrared radiation, which causes the substance to reach very high temperatures (750–930 ºC) in a short time. Micronization of grains and pulses causes the seeds to swell and rupture, reducing their cooking time and altering other physical and chemical properties. For example, micronization can improve pulses’ PER, indispensable amino acid score, and shelf stability, while decreasing undesirable attributes such as tannins, phytic acid, trypsin inhibitor and lipoxygenase activities, and oligosaccharides.
The researchers added micronized or non-micronized green lentil flour to ground meat at levels of 5–12% total volume. Wheat flour, which is a common binding agent, is typically added at 5%. The team found that the micronized lentil flour was better at maintaining meat redness and delaying the oxidation of pigments and lipids than the non-micronized flour. The lentil flour also provided protein and contributed to increased meat firmness and juiciness. “The micronized lentils brought a nice nutty flavor to the product,” adds Wanasundara.
Osen and colleagues used sweet lupine to formulate a refreshing, protein-rich beverage (Fraunhofer Institute, https://tinyurl.com/lupine-drink, 2017) (Fig. 4). Although lupine is perhaps best known as an ornamental flower, the plant’s seeds are a traditional pickled snack food in some parts of the world, such as the Mediterranean basin and Latin America. Osen chose lupine for the protein-rich beverage because this pulse contains protein that dissolves in the acidic pH range, whereas most other proteins are not soluble at low pH.
A slightly acidic drink tastes refreshing. However, like other pulses, lupine contains phytic acid, which binds minerals and inhibits enzymes, limiting the drink’s digestibility. To reduce the phytic acid content, Osen and colleagues used a two-stage mashing and fermentation process, during which microorganisms degraded the phytic acid by hydrolysis. The result was a lupine protein isolate in the form of paste or powder, with a relatively neutral taste. Although the drink is non-alcoholic, the process is similar to beer brewing and can be conducted at any brewery.
FIG. 4: (A) Cultivated sweet lupine.(B) A refreshing, protein-rich drink made from the extract of sweet lupine. Credit: Fraunhofer IVV
Future challenges
Although pulses are poised to become a major food trend, there are still some issues that hinder their widespread use. Sensory attributes are one such challenge. Pulses generally impart an off-flavor to foods, ranging from “fruity” to “beany” to “earthy” to “bitter.” In some cases, these flavors may add to the appeal of the food, but in other cases, they are undesirable. Deflavoring methods are being investigated, and some have been patented by food companies. The details of such methods are usually proprietary but involve the removal or modification of volatile and nonvolatile compounds that are responsible for the undesirable sensory characteristics (Nosworthy, M.G., et al., http://dx.doi.org/10.1094/CFW-62-4-0139, 2017).
If pulse crops are to meet predicted future demands, agricultural innovation, which lags behind cereal and oilseed crops, must accelerate. Over the past few years, global pulse crop production has remained relatively stagnant in terms of yield per hectare, hectares planted, and total volume produced (Global Pulse Confederation, https://tinyurl.com/GPC-strategy, 2016). Pulse crops must be further optimized for yield and resistance to disease, pests, weeds, and drought. There are also opportunities to improve protein content and quality, minimize antinutrients, shorten cooking time, and improve sensory attributes.
“Protein quality is not a typical breeding target for pulses, but we’re trying to encourage that,” says House. “However, it’s very expensive and time-consuming to analyze amino acids, and when you’re a breeder, you’ve got thousands of samples to screen. We’re trying to give new tools to breeders so that they can more quickly assess amino acid composition in germplasm.”
Thus far, pulse producers have embraced classical breeding over genetic modification, perhaps to avoid the controversy that GMOs often generate in pulses’ target market (people seeking plant-based proteins and soy alternatives). Yet transgenic approaches and gene editing can rapidly accelerate the rate of trait discovery and development. “We’ll probably see some efforts using new technology, not necessarily transgenics, but perhaps gene editing approaches such as CRISPR technology, to make more rapid advancements in pulse breeding,” says House. However, he notes that the distinction between genetic modification and gene editing can be challenging to communicate to the average consumer.
According to Kerr, the infrastructure and manufacturing expertise for pulses are currently at the stage soy was at about 40 years ago. “Pulse crops are at a very exciting point in their development. There’s tremendous motivation for companies around the world to look at pulse crops as another protein-rich raw material that can be consumed in whole form or refined into various ingredients and then used in new food products,” says Kerr. “The pulse industry will need organizations like AOCS that have a long history of expertise in not only the biotechnology of the raw material, but also in the processing technology, nutrition science, and food chemistry needed to make and use these ingredients.”
Laura Cassiday is an associate editor of Inform at AOCS. She can be contacted at laura.cassiday@aocs.org.
Information
- Canadian Special Crops Association (2014) “Canadian Special Crops Association 2011-2014 strategic plan.”
- Cross, B. (2013) “Lentils give beef a boost to improve consumer appeal.” Western Producer, March 15, 2013.
- Dahl, W.J., et al. (2012) “Review of the health benefits of peas (Pisum sativum L.).” Br. J. Nutr. 108, S3¬–S10. https://doi.org/10.1017/S0007114512000852
- Elkowicz, K., and Sosulski, F.W. (1982) “Antinutritive factors in eleven legumes and their air-classified protein and starch fractions.” J. Food Sci. 47, 1301–1304. https://doi.org/10.1111/j.1365-2621.1982.tb07673.x
- Food and Agriculture Organization (FAO) of the United Nations (2016) Pulses: nutritional seeds for a sustainable future.
- Fraunhofer Institute (2017) “Perfect for athletes and health aficionados: the lupine protein beverage.” News release, January 8, 2017.
- Global Pulse Confederation (2016) “10-year research strategy for pulse crops.”
- Hughes, G.J., et al. (2011) “Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: criteria for evaluation.” J. Agric. Food Chem. 59, 12707–12712.
- Hui, A. (2017) “The ‘future of food.’” The Globe and Mail, December 31, 2017.
- Kim, S.J., et al. (2016) “Effects of dietary pulse consumption on body weight: a systematic review and meta-analysis of randomized controlled trials.” Am. J. Clin. Nutr. 103, 1213¬–1223. https://doi.org/10.3945/ajcn.115.124677
- Lee, W.T.K., et al. (2016) “Research approaches and methods for evaluating the protein quality of human foods proposed by an FAO expert working group in 2014.” J. Nutr. 146, 929–932. http://doi.org/10.3945/jn.115.222109
- Marinangeli, C.P.F., and House, J.D. (2017) “Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health.” Nutr. Rev. 75, 658–667. https://doi.org/10.1093/nutrit/nux025
- Marinangeli, C.P, and Jones, P.J. (2011) “Whole and fractionated yellow pea flours reduce fasting insulin and insulin resistance in hypercholesterolaemic and overweight human subjects.” Br. J. Nutr. 105, 110–117. https://doi.org/10.1017/S0007114510003156, 2011
- Nosworthy, M.G., and House, J D. (2017) “Factors influencing the quality of dietary proteins: implications for pulses.” Cereal Chem. 94, 49–57. http://dx.doi.org/10.1094/CCHEM-04-16-0104-FI
- Nosworthy, M.G., et al. (2017) “Determination of the protein quality of cooked Canadian pulses.” Food Sci. Nutr. 5, 896–903. http://dx.doi.org/10.1002/fsn3.473
- Nosworthy, M.G., et al. (2017) “Does the concentration, isolation, or deflavoring of pea, lentil, and faba bean protein alter protein quality?” Cereal Foods World 62, 139–142. http://dx.doi.org/10.1094/CFW-62-4-0139, 2017
- Pulse Canada Fact Sheet. “Pulses in pasta applications.”
- Pulse Canada Fact Sheet. “Using yellow pea flours to improve the glycemic profile of cereal products.”
- Sanjeewa, W.G.T., et al. (2010) “Characterization of chickpea (Cicer arietinum L.) flours and application in low-fat pork bologna as a model system.” Food Res. Internatl. 43, 617–626.
- Skerritt, J. (2018) “Canadian farmers ditch pulses for canola after pea boom goes bust.” Financial Post, February 14, 2018.
- Tzitzikas, E., N. et al. (2006) “Genetic variation in pea seed globulin composition.” J. Agric. Food Chem. 54, 425–433.
- Wyers, R. (2018) “Pea protein surge: Cargill invests in Puris, the largest North American producer.” Foodingredientsfirst.com, January 18, 2018.