Hydrocolloids get personal
By Laura Cassiday
June 2012
For decades, food manufacturers have added hydrocolloids such as xanthan gum, pectin, and carrageenan to give salad dressings, jams, ice creams, and other foods their characteristic textures. Hydrocolloids are substances that alter the flow properties, or rheology, of aqueous solutions in which they are dispersed. More recently, these food ingredients have found a fit in the personal care industry, where they often replace synthetic chemicals with similar properties. Driven by consumers’ thirst for “all-natural” products, many manufacturers are trying to leverage the long history of hydrocolloids in the food industry for personal care applications.
“Technically, there’s not much difference between Thousand Island salad dressing and facial moisturizer,” says Arthur Rich, a cosmetic chemist consultant and founder of A. Rich Development, LLC, in Chestnut Ridge, New York, USA. Hydrocolloids that make a salad dressing feel rich and creamy can impart similar characteristics to a moisturizer or body lotion. Hydrocolloids already appear in the ingredients lists of many lotions, creams, hair care products, shower gels, sunscreens, toothpastes, antiperspirants/deodorants, and cosmetics.
Hydrocolloid functions
Hydrocolloids are hydrophilic polymers derived from plant, animal, microbial, or synthetic sources. When added to water, hydrocolloids disperse evenly as microscopic particles. At sufficiently high concentrations, the polymers become entangled with each other, forming loose networks that change the flow and spread properties of solutions. Many hydrocolloids, such as gelatin and pectin, can form gels by hydrogen bonding within and between polymers. The structure, charge, and concentration of a hydrocolloid and its interactions with other ingredients determine the rheology of the solution.
In addition to acting as rheology modifiers, hydrocolloids serve other functions in personal care products. By surrounding oil droplets in oil-in-water emulsions, hydrocolloids can prevent the droplets from coalescing. This droplet stabilization helps prevent products from separating, settling, or clinging to their container and improves their heat resistance. Some amphiphilic hydrocolloids, such as acrylate copolymers, can actually penetrate the oil-water interface of a droplet. By reducing the interfacial tension, amphiphilic hydrocolloids can make it easier to produce small oil droplets in an aqueous solution.
Another important function of hydrocolloids is to reduce the tendency of a fluid to thin upon agitation—the so-called thixotropic behavior. Reducing thixotropy is particularly important for sunscreens, says Holger Seidel, senior technical service manager at Azelis Kosmetik, GmbH, which is based in Moers, Germany. Azelis is the leading pan-European distributor of specialty chemicals, including hydrocolloids.

“When you apply sunscreen to your skin, the formulation breaks down immediately,” says Seidel. “If the structure is not able to recover very quickly, then the sunscreen will flow into your wrinkles, and your skin will not be completely protected.” Hydrocolloids help the sunscreen emulsion recover its structure after application, allowing uniform and long-lasting protection from the sun.
Hydrocolloids also make formulations more aesthetically pleasing. “Hydrocolloids allow your toothpaste to be extruded from the tube with a lovely shape and stay on your brush,” says Marie-Laure Roumiguière, pharma and personal care category manager in Paris, France, at Cargill Texturizing Solutions, an international producer of texturizers and emulsifiers. Hydrocolloids can help suspend millimeter-sized particulates, such as luffa sponges or plastic beads, in exfoliating cleansers, or enhance a cleanser’s foaming action. They also contribute to the “skin feel” of a cream or lotion—whether the product feels rich, creamy, sticky, slippery, or stringy when applied to the skin. Some hydrocolloids used in moisturizers form films on the skin surface. “When the product dries down, the hydrocolloid leaves a film that inhibits the evaporation of moisture from the skin,” explains Rich. Many hair care products contain hydrocolloids as lubricants. “Hair is in one of its most vulnerable states when it’s wet,” says Jeni Thomas, principal scientist and scientific communications manager at Procter & Gamble (P&G) in Cincinnati, Ohio, USA. “Using hydrocolloids is a great way to provide the lubrication that helps keep hair protected from breakage during washing and wet combing.”
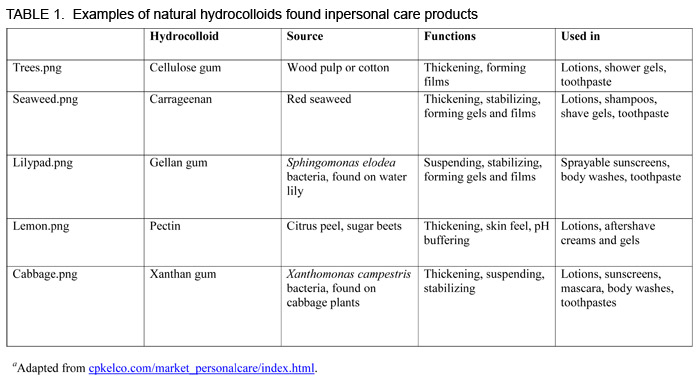
Hydrocollloid sources
Common synthetic hydrocolloids are acrylic acid polymers, also known as carbomers. Natural hydrocolloids are derived from plant (pectin, carrageenan, cellulose gum, locust bean gum), animal (gelatin), or microbial (xanthan gum, gellan gum) sources.

“If you’ve ever had old cabbage in your refrigerator that gets a bit slimy, there’s actually a bacterium, Xanthomonas campestris, that consumes the natural sugar of the cabbage and produces a protective gum, which is in essence xanthan gum,” says Bruce Hein, global positioning manager at CP Kelco, an international producer of specialty hydrocolloids with headquarters in Atlanta, Georgia, USA. CP Kelco’s facilities ferment X. campestris on an industrial scale. They then purify and further process the xanthan gum into special grades that impart specific rheological properties.
Some chemical manufacturers have combined science with nature to generate semisynthetic hydrocolloids. For example, chemists produce cellulose gum by adding carboxymethyl groups to the backbone of cellulose, a polysaccharide derived from wood pulp or cotton. The carboxymethyl group imparts a negative charge to cellulose and makes it water soluble. Specific modifications to the cellulose backbone influence the rheology and thixotropy of the solution.
Synthetic hydrocolloids offer certain advantages over their natural counterparts, such as increased potency, resistance to microbial degradation, and solution clarity. However, many manufacturers of personal care products are trying to replace synthetic hydrocolloids with natural ones in order to satisfy consumer demand. “There’s a trend in the personal care industry to move away from the more chemical-sounding products to food ingredients that are more familiar to the consumer,” says Hein.
“Natural” hydrocolloids
According to a 2011 research study published by Market Publishers Ltd., the US consumer market for natural and organic skin care, hair care, and makeup boomed 61%—to $7.7 billion—from 2005 to 2010. As a result, many manufacturers are jumping on the all-natural bandwagon, seeking a “green” stamp of approval from natural-product-certifying agencies such as ECOCERT and the Natural Products Association.
However, the standard for what constitutes “natural” can vary greatly among certifying agencies, particularly with regard to semisynthetic ingredients. “There’s no strict definition of ‘natural’ in the personal care industry,” says Phillip Mitteness, Technical Development Manager at Univar, a global chemical distributor with a personal care industry focus, headquartered in Redmond, Washington. USA. “Many personal care companies are looking to certifying agencies to provide them with a definition.”
But for personal care companies eager to go green, finding a natural replacement for a synthetic hydrocolloid isn’t trivial. “When you switch from a synthetic hydrocolloid to a natural version, the product appearance completely changes,” says Seidel. “You don’t have the same flow properties, you may have a lumpy appearance, and the formulation is no longer crystal clear.”
“There’s typically a tradeoff between having a natural product, and aesthetics or performance,” agrees Mitteness. “And of course, the price goes up for natural products.”
Some manufacturers try to grasp the best of both worlds by using semisynthetic hydrocolloids, which they can advertise as being “naturally derived.” In 2009, P&G launched the Pantene® Nature Fusion hair care collection. Key to the line’s marketing approach is that Nature Fusion products contain a chemically modified version of cassia gum, a hydrocolloid from the seeds of a legume native to India. Pantene scientists found that adding hydroxypropyltrimonium cations to the cassia polysaccharide enhanced its hair-conditioning properties.
In Nature Fusion shampoo, the positively charged cassia polymer interacts with anionic surfactants to form a conditioning complex, or coacervate. “The coacervate is a very, very hydrated complex that separates out of the rinse water and deposits on the hair fiber surface,” says Thomas. Even after rinsing, the modified hydrocolloid lubricates hair, minimizing friction and preventing breakage during wet combing.
Cationic polymers such as the modified cassia polymer play a different role in conditioners. “Hair has a negative charge, and the more damaged it is, the more negative charge it takes on,” explains Thomas. “In conditioners, the electrostatic interaction between cationic polymers and the negative charges on hair helps reinforce weakened areas on the hair fiber.” This interaction not only helps strengthen hair but also reduces static charge buildup, says Thomas.
Product formulation
Another trend in the personal care industry is a move toward low-sulfate or sulfate-free cleansers. Sulfates such as sodium lauryl sulfate are efficient emulsifiers, but they can irritate sensitive skin. Some hydrocolloids, such as hydroxypropyl methylcellulose, can perform double duty as emulsifiers. And because most hydrocolloids are large polymers, they are less likely to penetrate the skin and cause irritation.
Given all the variables that go into product formulation, selecting the appropriate hydrocolloid for a particular application can be challenging. Fortunately, many chemical suppliers and distributors offer extensive technical support for their products. According to Seidel, the technical service department at Azelis Kosmetic Germany GmbH fields about 700 technical requests per month from Germany, Austria, and Switzerland alone, some of them involving hydrocolloids. The company has a well-equipped application lab where researchers develop prototype formulas and assist customers with their technical service requests.
CP Kelco offers customers a personal care demo kit, which highlights the use of the company’s hydrocolloids in product samples such as sunscreen, shower gel, hand sanitizer, body lotion, and a seaweed gel mask. The company provides the recipe for each prototype formula, including the type and amount of hydrocolloid, which gives customers a baseline for formulating their own products. Other companies with marketing and technical capabilities, including Univar and Azelis, have likewise developed prototypes to assist customers in product formulation.
Ultimately, the selection of hydrocolloid depends on the desired properties of the personal care product. When choosing among the many hydrocolloids on the market, formulation chemists must consider the product’s rheology, skin feel, shelf life, compatibility with other ingredients, pH range, delivery method, and whether the product is to be marketed as “natural.” In addition, manufacturers and consumers alike are increasingly concerned about the sustainability of the ingredients and the environmental footprint of the production process.
Sometimes, a mixture of hydrocolloids functions better in a product than either one in isolation. “If you mix xanthan gum and carrageenan, you get different flow characteristics than you would with either separately,” says Mitteness. “And when you combine two hydrocolloids in different ratios, the properties change as well.”
Because the natural products movement shows no signs of losing steam, consumers are increasingly likely to recognize ingredients from salad dressing or ice cream in their moisturizer or shower gel. “It’s very exciting to translate our food expertise in texturizing solutions to the personal care industry,” says Roumiguière. Although the long history of food hydrocolloids provides a solid foundation, the unique demands of a wide array of personal care products will likely require innovation in the form of new hydrocolloid sources, mixtures, and modifications.
Hydrocolloids get personal (.pdf)
Laura Cassiday is a freelance science writer and editor based in Hudson, Colorado, USA. She has a Ph.D. in biochemistry from the Mayo Graduate School and can be contacted at lauracassiday@yahoo.com.
