The mysterious case of the arsenolipids
By Laura Cassiday
March 2017
- Arsenolipids are lipid-soluble compounds that contain arsenic and a long aliphatic chain. Made by algae from inorganic arsenic in seawater, arsenolipids accumulate in fish and other marine animals.
- In recent years, about 50 arsenolipids have been identified, including arsenic-containing hydrocarbons, fatty acids, phospholipids, phosphatidylcholines, and fatty alcohols. Many more likely remain to be discovered.
- The physiological role of arsenolipids in algae and their effects on marine creatures and humans are largely unknown.
In Victorian thrillers, murderers often dispatched their victims with a large dose of arsenic stirred into hot tea or sprinkled onto food. The perfect poison, arsenic mimicked symptoms of natural diseases such as cholera and was difficult to detect with the forensic techniques of the era. In more recent times, low levels of arsenic have been discovered in drinking water, rice, and seafood. Whereas the arsenic in water and rice is in the inorganic form, in fish and seaweed a substantial portion of the arsenic is sequestered in intriguing compounds called arsenolipids. As yet, very little is known about the biosynthesis, metabolism, function, or toxicity of arsenolipids. Although their levels in seafood and fish oils are unlikely to pose a risk to human health, some researchers believe that it is nonetheless important to better characterize arsenolipids, not only to guide toxicological risk assessments, but also to gain an improved understanding of arsenic cycling in the environment.
Fat-soluble arsenic
The presence of arsenic in marine creatures is not surprising given that arsenic is a ubiquitous component of seawater, occurring at fairly uniform levels of 0.5–2 μg/L worldwide (Sele, V., et al., http://dx.doi.org/10.1016/j.foodchem.2012.02.004, 2012). Most inorganic arsenic is released into the ocean by natural processes, such as volcanoes and weathering of minerals, although local arsenic contamination from man-made sources, such as coal burning, pesticides, or ore smelting, can occur. In the late 19th century, arsenic had already been detected in fish. However, most studies since then have focused on water-soluble arsenic compounds, such as inorganic arsenic and arsenobetaine (trimethylarsonioacetate), an organic small molecule that usually comprises more than 80% of the total arsenic in marine organisms.
On the other hand, lipid-soluble arsenic compounds have proven much more difficult to isolate and analyze. In 1968, in the Journal of the American Oil Chemists’ Society (JAOCS), Lunde published the first report of lipid-soluble arsenic compounds, proposed to be arsenic-containing phospholipids, in cod liver and herring oils. Twenty years later, Morita and Shibata accomplished the first structural identification of an arsenolipid—a phospholipid with an arsenosugar head group, from a brown alga (Chemosphere, 1988).
Since then, about 50 different arsenolipids have been identified, estimates Jörg Feldmann, professor of environmental analytical chemistry and director of the Trace Element Speciation Laboratory at the University of Aberdeen, in Scotland. Despite this progress, the arsenolipids are still not well characterized or studied. “I think in the world there are only really three or four groups doing arsenolipid research right now,” says Feldmann. “When we started looking at arsenolipids 10 or 15 years ago, there were no real methods available to extract the arsenolipids and analyze them without changing them.”
“Many arsenolipids have been identified, but there are many more that haven’t been identified,” says Kevin Francesconi, professor in analytical chemistry at the University of Graz, in Austria. “Most of them are present at really trace amounts. There are probably 10 compounds that make up 80–90% of the total amount of arsenolipids, I would estimate.”
Types of arsenolipids
The definition of an arsenolipid depends, to some extent, on whom you ask. According to William Christie, consultant and professor emeritus at the James Hutton Institute in Dundee, Scotland, some researchers define an arsenolipid as any arsenic-containing compound that is soluble in organic solvents. “Some people look at things like trimethylarsine, a very simple molecule, and call that a lipid,” he says. “If you do that, there are literally hundreds of different arsenolipids, so I restrict myself to those that contain a long aliphatic chain.” Using this definition, five main groups of arsenolipids have been identified: arsenic-containing hydrocarbons (AsHCs), fatty acids (AsFAs), phospholipids, phosphatidylcholines, and fatty alcohols (Fig. 1).
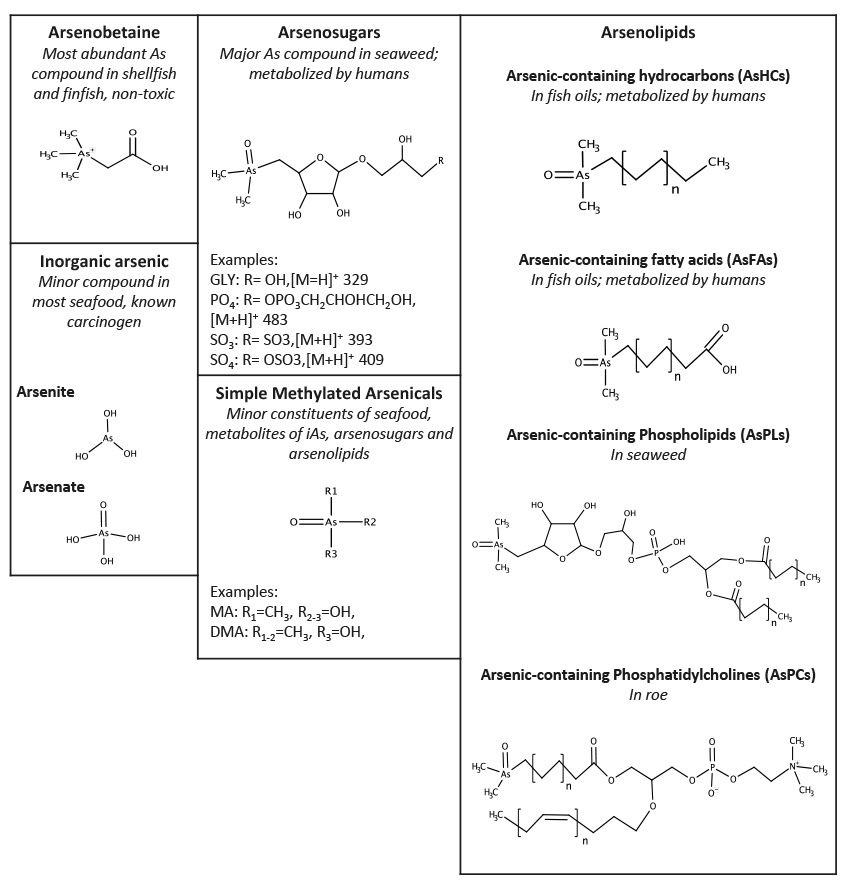
FIG. 1. Arsenic compounds found in seafood. Not shown in the arsenolipid column are the arsenic-containing fatty alcohols, which are similar to the AsHCs, but with a positively charged terminal trimethylarsonium group at one end and a hydroxyl group at the other. Reprinted with permission from Total Environ. Epub ahead of print. Taylor, V., et al. (2016) "Human exposure to organic arsenic species from seafood." http://dx.doi.org/10.1016/j.scitotenv.2016.12.113.
The AsHCs are long-chain aliphatic compounds with a terminal dimethylarsinoyl group. Odd- and even-numbered species of AsHCs have been identified, with up to 19 carbons and up to 7 double bonds (AOCS Lipid Library, http://tinyurl.com/arsenolipids). AsHCs have been detected in brown algae, fish, and fish oils. In 2013, Feldmann and colleagues identified seven different AsHCs in oil from the capelin (Mallotus villosus) fish (Amayo, K. O., et al., http://dx.doi.org/10.1021/ac4020935). Three of these comprised the major arsenolipids in the capelin oil, together accounting for more than 80% of the total arsenic content.
Arsenic-containing phospholipids are analogous to regular phospholipids, but with arsenic in the head group. For example, phosphatidylarsenocholine, identified in algae, contains arsenic in place of the nitrogen atom in the choline head group. Many arsenic-containing phospholipids contain an arsenosugar in place of the choline group. Related compounds, the arsenic-containing phosphatidylcholines, were recently identified by Francesconi’s group in herring caviar (Viczek, S. A., et al., http://dx.doi.org/10.1002/anie.201512031, 2016). These phospholipids have a normal choline head group, but an AsFA as one of the fatty acids in their tail.
In 2013, Feldmann’s group identified two arsenic-containing fatty alcohols in capelin oil (Amayo, K. O., et al., http://dx.doi.org/10.1021/ac4020935). The compounds, present at low concentrations, were alcohols with long hydrocarbon chains and a positively charged terminal trimethylarsonium group. “So far we’re the only ones who have found arsenic-containing fatty alcohols, and it was quite tricky to do that,” says Feldmann. “You need some derivatization methods, and that’s maybe why they are so elusive.”
Arsenolipid analysis
The characterization of arsenolipids has proven challenging for several reasons. In comparison to regular cellular lipids, arsenolipids are present at low concentrations, making identification and quantification difficult. Care must be taken so that labile arsenolipids are not hydrolyzed during sample preparation and analysis. Analytical techniques for water-soluble arsenic species are not appropriate for the analysis of lipid-soluble species. Also, there are currently no commercially available arsenolipid standards or certified reference materials, making inter-laboratory comparisons difficult (Taylor, V., et al., http://dx.doi.org/10.1016/j.scitotenv.2016.12.113, 2016).
In the past, arsenolipids could only be analyzed indirectly. HPLC/ICP-MS (high-performance liquid chromatography/inductively coupled plasma-mass spectrometry) is a powerful technique that can be used to detect trace elements, such as arsenic, in samples. Initially, however, the technique could be used only for water-soluble arsenic species. So researchers were forced to chemically or enzymatically hydrolyze arsenolipid samples into water-soluble fragments, analyze the fragments by HPLC/ICP-MS, and then deduce the structures of the original compounds (Amayo, K. O., et al., http://dx.doi.org/10.1021/ac4020935).
In the early 2000s, HPLC/ICP-MS was modified to allow for the direct analysis of arsenolipids. To separate arsenolipids by HPLC, an organic mobile phase is needed, which can destabilize the plasma ionization source of the original ICP-MS. To accommodate organic mobile phases, a modified ICP-MS set-up using low solvent flows and adding oxygen to the nebulizer gas is necessary (Sele, V., et al., http://dx.doi.org/10.1016/j.foodchem.2012.02.004, 2012). This improvement has greatly accelerated the rate of arsenolipid discovery in the past few years.
There are four main steps to arsenolipid analysis: extraction, separation, detection, and characterization (Taylor, V., et al., http://dx.doi.org/10.1016/j.scitotenv.2016.12.113, 2016). The extraction generally involves stepwise solvent partitioning to separate arsenic-containing compounds from the overall lipid matrix. For example, Francesconi and coworkers partitioned the lipid-soluble fraction from sashimi-grade tuna fish between hexane and aqueous methanol (Taleshi, M. S., et al., http://dx.doi.org/10.1021/es9030358, 2010). Because many arsenolipids contain a polar (e.g., dimethylarsinoyl) group, they tend to accumulate in the aqueous methanol layer. The non-polar hexane extract contained more than 90% of the total lipids. In contrast, the polar methanol extract contained only 5% of the total lipid, but 50% of the arsenic. Therefore, the researchers analyzed the methanol extract, which was enriched in arsenolipids, by HPLC/ICP-MS and HPLC/electrospray ionization (ESI)-MS/MS. They identified two AsHCs that together accounted for about 40% of the total fat-soluble arsenic in the tuna.
In a more recent study, Francesconi and colleagues extracted the hexane layer from blue whiting oil with aqueous isopropanol to yield a “less-polar” lipid fraction that had a polarity in between the polar (hexane) and nonpolar (methanol) extracts (Taleshi, et al., http://dx.doi.org/10.1021/es9030358, 2014). Increasing chain length reduces the polarity of arsenolipids, whereas functional groups or unsaturation can increase polarity. This strategy allowed the researchers to identify 4 novel AsHCs from the fish oil.
Reversed-phase HPLC is the most common separation step used for arsenolipid analysis. Polar compounds elute from the chromatography column more rapidly than less-polar compounds. In this way, the arsenolipids from a fraction can be further separated so that they can be detected sequentially by ICP-MS. ICP-MS has excellent detection limits and a linear range capable of quantitating compounds at concentrations ranging from low ng/L to 1 mg/L within the same run (Taylor, V., et al., http://dx.doi.org/10.1016/j.scitotenv.2016.12.113, 2016), provided the compounds are well separated by HPLC.
The characterization step typically involves comparing the ICP-MS data with molecular mass and fragmentation data from ESI-MS/MS to assign structures to arsenolipids. “We split the flow from the HPLC column, and we use both elemental mass spectrometry [ICP-MS], which enables us to see all the compounds that contain arsenic, and molecular mass spectrometry with a high-resolution instrument [ESI-MS/MS],” says Francesconi. “If we see a peak corresponding to an arsenic compound, we are also very often able to get the molecular mass of that compound by using the two forms of detection simultaneously. Once we have the mass and fragmentation pattern, we can start putting a structure forward.” Often, the researchers will then chemically synthesize the proposed molecule and analyze it to prove that the structure is correct.
Mysterious molecules
Although much progress has been made in identifying arsenolipids, their origins and interactions are still enigmatic. Arsenate [(H2AsO4)-], the predominant form of arsenic in seawater, is readily taken up by algae (Fig. 2). Because arsenate is structurally similar to phosphate [(H2PO4)-], the algae’s phosphate transporters may not be able to differentiate between the two anions. Once inside the algae, arsenate undergoes a series of biotransformations that result in a variety of organoarsenic compounds, including arsenosugars and arsenolipids. “We know that algae make arsenolipids,” says Francesconi. “Whether fish make them de novo from inorganic arsenic, I think that’s less likely. We think that the algae are the starting points, and the fish accumulate, but also modify, the arsenolipids that they ingest.”

FIG. 2. Seaweeds, or brown algae, produce arsenolipids, which accumulate in fish and other marine creatures that ingest the seaweed. Credit: Stef Maruch, via Wikimedia Commons
In marine animals and algae, total arsenic concentrations range from 1–100 mg/kg, with considerable variations between and within species (Sele, V., et al., http://dx.doi.org/10.1016/j.foodchem.2012.02.004, 2012). Reports of total arsenic levels in commercial fish oils have ranged from 0.2¬–16 mg/kg, depending on the fish species. For comparison, terrestrial samples usually contain much lower levels of arsenic (below 0.02 mg/kg), with the exception of rice, which accumulates 0.02–1 mg/kg total arsenic. Although much of the arsenic in rice is in the inorganic form, marine animals typically contain low levels of inorganic arsenic. About 10–30% of the arsenic in marine animals is in the form of lipid-soluble arsenic compounds. In brown algae, however, arsenolipids can account for more than 50% of the total arsenic.
When algae take up arsenate, which is highly toxic, they rapidly detoxify it by adding two methyl groups to produce dimethylarsinate [DMA; Me2As(O)O-]. Many organisms, including humans, can likewise convert arsenate into DMA. However, unlike humans, marine algae use DMA as an intermediate to produce a wide range of arsenolipids (Francesconi, K. A., and Schwerdtle, T., http://dx.doi.org/10.1002/lite.201600024, 2016).
An unanswered question is why algae produce arsenolipids. One possibility is that, like the phosphate transporters, biosynthetic enzymes mistake arsenic-containing compounds for other molecules, and then use them as building blocks for cellular lipids. If this were the case, the arsenolipids in marine organisms would likely reflect the natural abundance of non-arsenic-containing lipids. Although more research is needed, abundant fatty acids such as palmitic and stearic acids are indeed more likely to appear as arsenolipids than less abundant fatty acids (Francesconi, K. A., and Schwerdtle, T., http://dx.doi.org/10.1002/lite.201600024, 2016). Another idea is that arsenolipid production could help with detoxification. Adding arsenic to lipids may facilitate its diffusion through the membrane and out of the cell.
An intriguing possibility is that, instead of being mere accidents or detoxification products, arsenolipids might actually serve a useful purpose in marine organisms. The early ocean, like now, contained appreciable amounts of arsenic. “Arsenic was always available, and usually life forms use all of the elements available at higher concentrations in their chemistry,” says Feldmann. “Arsenic chemistry is distinctively different from any other chemistry. Therefore, there’s a gut feeling that arsenic is somehow an essential element at a low concentration.”
When Feldmann and colleagues challenged brown algae in the lab with different forms of environmental stress (low nitrate, low phosphate, oxidative stress), the algae responded by making more arsenolipids (Pétursdóttir, Á. H., et al., http://dx.doi.org/10.1071/EN14229, 2016). Low-phosphate conditions preferentially increased the biosynthesis of arsenic-containing phospholipids. Therefore, it is possible that arsenolipids are an adaptive mechanism to certain forms of environmental stress.
The presence of arsenic-containing phospholipids in cell membranes may alter their structure or fluidity, which in turn could affect cell growth, signaling, and ligand-receptor interactions (Sele, V., et al., http://dx.doi.org/10.1016/j.foodchem.2012.02.004, 2012). However, arsenic-containing phospholipids would not normally be expected to have large effects on membranes because their concentration would be much lower than that of regular phospholipids. Perhaps the contribution of arsenolipids to membranes would become more important for algae living under phosphorus-deficient conditions.
“You can do various experiments to suggest that algae are quite happy with low levels of arsenic, and some grow even better when there’s a little bit more arsenic in the water,” says Francesconi. “It’s certainly a fascinating topic, but we haven’t got any firm evidence to say, yes, arsenic is useful, and it is being used by some organisms.”
Cause for concern?
Chronic exposure to inorganic arsenic in drinking water can have serious health effects, including skin, bladder, and lung cancers. The International Agency for Research on Cancer (IARC) has classified inorganic arsenic as a Group 1 human carcinogen. Seafood is one of the major contributors of arsenic in the diet (more than 90% in the United States); however, most arsenic in seafood is present as organoarsenic compounds with largely unknown health effects (Taylor, V., et al., http://dx.doi.org/10.1016/j.scitotenv.2016.12.113, 2016).
The major organoarsenic compound in seafood, arsenobetaine, is known to be nontoxic. Arsenobetaine is metabolically inert, meaning that it is excreted unchanged from the human body in the urine. In contrast, arsenolipids and arsenosugars are metabolized by humans, mainly to DMA—an IARC Group 2B (possible) carcinogen, and the same metabolite produced upon ingestion of inorganic arsenic. There is also a concern that arsenolipids could produce toxic intermediates along the pathway to DMA.
To gain a better understanding of human metabolism of arsenolipids, Francesconi and coworkers performed a small study in which two male volunteers ingested canned cod liver (Schmeisser, E., et al., http://dx.doi.org/10.1007/s00216-006-0401-x, 2006). Arsenic metabolites in the volunteers’ urine were monitored by HPLC/ICP-MS for 66 hours afterwards. As expected, the arsenobetaine from the cod liver samples was excreted in the urine unchanged. The arsenolipids, however, were metabolized. For both volunteers, the most abundant arsenolipid metabolite was DMA, followed by four novel, short-chain AsFAs. The peak arsenic concentration in urine occurred between 7 and 15 hours, and the AsFAs reached background levels by 24 h after ingestion in both volunteers. By the end of the experiment, about 90% of the ingested arsenic had been accounted for in the urine of both volunteers. This preliminary study suggests that ingested arsenolipids are rapidly metabolized to water-soluble compounds and excreted. The time profile indicates that arsenolipids are first metabolized to AsFAs, and then further to DMA
In an in vitro study, Schwerdtle, Francesconi, and colleagues investigated the cellular toxicity of AsHCs in cultured human bladder and liver cells (Meyer, S., et al., http://dx.doi.org/10.1039/c4mt00061g, 2014). Surprisingly, the cytotoxicity of the three tested AsHCs was comparable to that of inorganic arsenic, in the low μM range. However, the mechanism of toxicity appeared to differ between the two arsenic forms: AsHCs reduced the cellular energy (ATP) level, rather than causing DNA damage as inorganic arsenic did. The AsHCs readily accumulated in the cultured cells, where they were localized in the membranous structures. The authors concluded that the study “cannot exclude a risk to human health related to the presence of arsenolipids in seafood, and indicates the urgent need for further toxicity studies in experimental animals to fully assess this possible risk.”
In a similar study with AsFAs, the same researchers found that AsFAs and three of their proposed water-soluble metabolites (DMA, DMAPr, and thio-DMAPr) were less cytotoxic than inorganic arsenic or AsHCs (Meyer, S., et al., http://dx.doi.org/10.1039/c5tx00122f, 2015). The AsFAs and DMA had cytotoxic effects at concentrations of about 50 μM and higher, whereas the other two metabolites were not cytotoxic. The researchers concluded that the tested AsFAs were 10–20-fold less toxic than AsHCs in the same cell lines. Because AsHCs are amphiphilic, they may be able to interact with cell membranes better than AsFAs, which have two polar end groups.
To study the fate of an ingested AsHC in a model organism, Karst, Francesconi, and coworkers used elemental laser ablation (LA)-ICP-MS and matrix-assisted laser desorption/ionization (MALDI)-MS to image and quantify the uptake of an AsHC in fruit flies (Drosophila melanogaster) (Niehoff, A.-C., et al., http://dx.doi.org/10.1021/acs.analchem.6b00333, 2016). The researchers fed larvae or adult flies either inorganic arsenic or an AsHC (1-dimethylarsinoylpentadecane) and then examined arsenic accumulation at different stages of development. The distribution patterns of inorganic arsenic and the AsHC varied substantially, and both larvae and adult flies accumulated higher concentrations of the AsHC than the inorganic arsenic. Disturbingly, perhaps, the AsHC was able to cross the blood-brain barrier and enter the brain of adult flies (in contrast to inorganic arsenic, which was not detected in the brain) (Fig. 3).
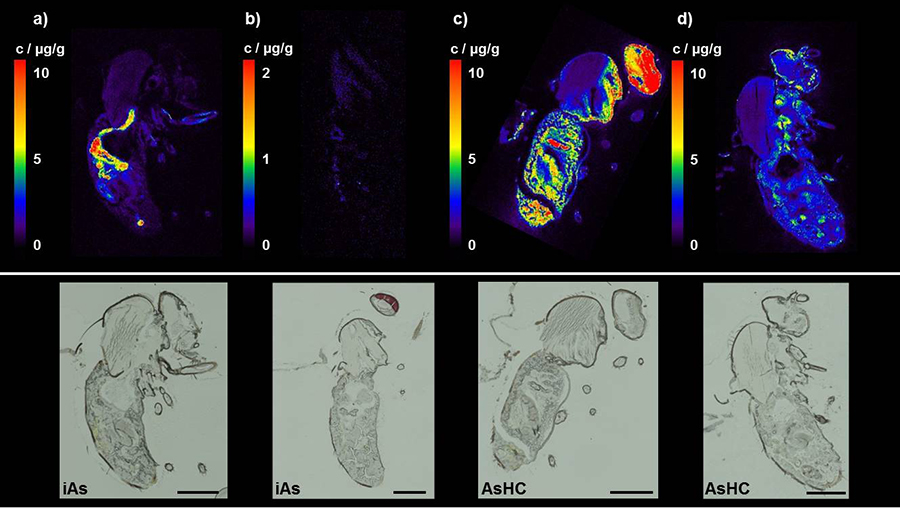
FIG. 3. Arsenic distribution in D. melanogaster adult flies after feeding of inorganic arsenic (iAs) or an AsHC for 3 days, followed by 2 days without arsenic-containing feed. LA-ICP-MS images of the 75As distribution in adult flies fed for 3 days with 50 μM (a) inorganic arsenic (arsenite), (c) AsHC-spiked feed (3.2 μg As/g), and (b, d) subsequent feeding without arsenic for 2 days, respectively. Microscopic images of the analyzed sections are shown for comparison. Scale bars, 500 μM. Reprinted with permission from Niehoff, A. C., et al. (2016) "Imaging by elemental and molecular mass spectrometry reveals the uptake of an arsenolipid in the brain of Drosophila melanogaster." Anal. Chem. 88, 5258-5263. http://dx.doi.org/10.1021/acs.analchem.6b00333. Copyright 2016 American Chemical Society
In 2009, the European Food Safety Authority (EFSA) concluded that a risk assessment of arsenolipids in seafood is urgently needed. Currently, regulations exist only for inorganic arsenic in drinking water and food. “We finally have legislation now for inorganic arsenic in rice, and I think the next big topic is really the arsenolipids,” says Feldmann. “But we still need to convince the analytical community to use all their power to do the identification. We need more than a handful of groups working on this topic. Since it’s in our food, we should be concerned about it.”
However, at the moment there is no cause for panic, or even for reducing fish consumption, in response to the emerging research on arsenolipids. Indeed, fish consumption in general is linked to positive health outcomes, such as reduced mortality from coronary heart disease, and neurological benefits. “Anything that has arsenic is potentially troublesome, but it’s too easy to exaggerate the risk,” says Christie. “People say, ‘Oh, arsenic, it’s a deadly poison,’ but as poisons go, it’s not that bad. It very much depends on the form of arsenic that is presented. So while people are right to keep an eye open, it doesn’t do to overstate the risk.”
Laura Cassiday is an associate editor of INFORM at AOCS. She can be contacted at laura.cassiday@aocs.org.
INFORMation
- Amayo, K. O., et al. (2013) “Novel identification of arsenolipids using chemical derivatizations in conjunction with RP-HPLC-ICPMS/ESMS.” Anal. Chem. 85, 9321–9327. http://dx.doi.org/10.1021/ac4020935.
- Amayo, K. O., et al. (2014) “Arsenolipids show different profiles in muscle tissues of four commercial fish species.” J. Trace Elem. Med. Biol. 28, 131–137. http://dx.doi.org/10.1016/j.jtemb.2013.11.004
- Francesconi, K. A., and Schwerdtle, T. (2016) “Fat-soluble arsenic—new lipids with a sting in their tail.” Lipid Technol. 28, 96¬–98. http://dx.doi.org/10.1002/lite.201600024
- Christie, W. (2014) “Arsenolipids.” AOCS Lipid Library, http://tinyurl.com/arsenolipids Lunde, G. (1968) “Analysis of arsenic in marine oils by neutron activation. Evidence of arseno organic compounds.” J. Am. Oil Chem. Soc. 45, 331–332.
- Meyer, S., et al. (2014) “In vitro toxicological characterization of three arsenic-containing hydrocarbons.” Metallomics 6, 1023–1033. http://dx.doi.org/10.1039/c4mt00061g
- Morita, M., and Shibata, Y. (1988) “Isolation and identification of arseno-lipid from a brown alga, Undaria Pinnatifida (Wakame).” Chemosphere 17, 1147–1152.
- Niehoff, A. C., et al. (2016) “Imaging by elemental and molecular mass spectrometry reveals the uptake of an arsenolipid in the brain of Drosophila melanogaster.” Anal. Chem. 88, 5258–5263. http://dx.doi.org/10.1021/acs.analchem.6b00333
- Pétursdóttir, Á. H., et al. (2016) “Environmental effects on arsenosugars and arsenolipids in Ectocarpus (Phaeophyta).”Environ. Chem. 13, 21–33. http://dx.doi.org/10.1071/EN14229
- Schmeisser, E., et al. (2006) “Human metabolism of arsenolipids present in cod liver.” Anal. Bioanal. Chem. 385, 367–376. http://dx.doi.org/10.1007/s00216-006-0401-x
- Sele, V. et al. (2012) “Arsenolipids in marine oils and fats: A review of occurrence, chemistry and future research needs.” Food Chem. 133, 618–630. http://dx.doi.org/10.1016/j.foodchem.2012.02.004
- Taylor, V., et al. (2016) “Human exposure to organic arsenic species from seafood.” Sci. Total Environ., Epub ahead of print. http://dx.doi.org/10.1016/j.scitotenv.2016.12.113
- Taleshi, M. S., et al. (2010) “Arsenic-containing lipids are natural constituents of sashimi tuna.” Environ. Sci. Technol. 44, 1478–1483. http://dx.doi.org/10.1021/es9030358
- Taleshi, M. S., et al. (2014) “Arsenolipids in oil from blue whiting Micromesistius poutassou — evidence for arsenic-containing esters.” Sci. Rep. 4, 7492. http://dx.doi.org/10.1038/srep07492
- Viczek, S. A., et al. (2016) “Arsenic-containing phosphatidylcholines: a new group of arsenolipids discovered in herring caviar.” Angew. Chem. Int. Ed. 55, 5259–5262. http://dx.doi.org/10.1002/anie.201512031
