Rethinking plastic packaging
By Rebecca Guenard
January 2019
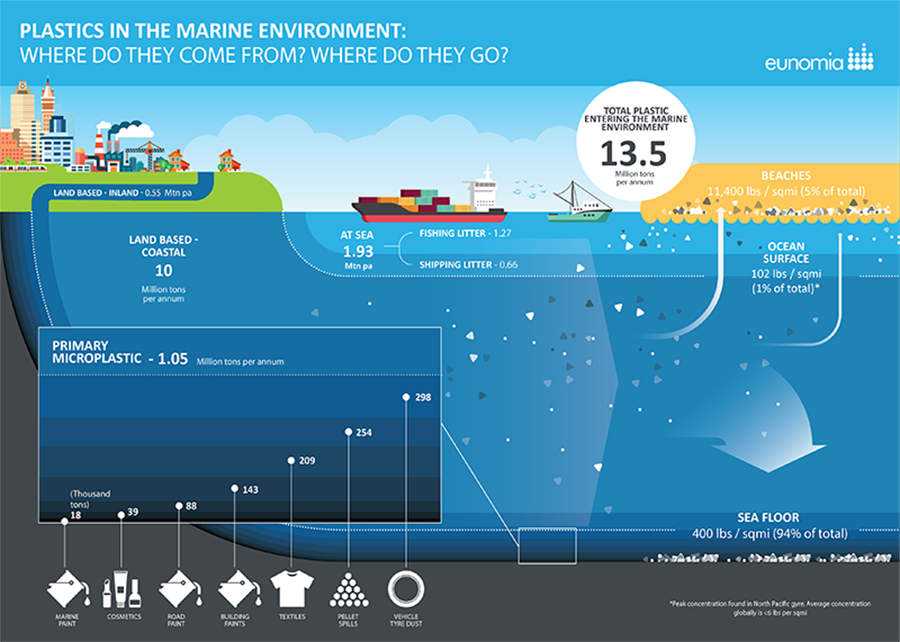
This infographic depicting sources of plastics in the marine environment is reprinted with permission from, "Plastics in the Marine Environment," a June 2016 report by Eunomia (http://www.eunomia.co.uk/reports-tools/plastics-in-the-marine-environment/).
- Corporations that use plastic packaging are investing in programs that reduce the potential for it to become marine waste, while also evaluating more sustainable ways to package their products.
- Packaging made of bio-based polymers could potentially reduce plastic waste, but natural starting materials do not necessarily lead to bio-gradable plastic, and bio-based products currently have a minor share of the plastics market.
- Researchers are exploring new chemistry to expand the recycling capabilities of plastic, for example, polymers that decompose back to their original monomer.
The United Nations reports that 8 million tons of plastic leak into the sea each year, the equivalent of a dump truck’s worth a minute. At this rate, in another 40 years there will be more plastic than fish in the sea (https://tinyurl.com/UN-ocean-plastics).
The issue has at last reached a tipping point, and organizations are taking action. The European parliament recently approved a ban on single-use plastics that will become effective within the next two years (https://tinyurl.com/yas9vof6). However, the American Chemistry Council argues that plastic is too vital a material for an outright ban (https://tinyurl.com/y7lh2mxm). Plastic provides safety in the form of bike helmets and air bags. It allows the medical field to maintain the sterilization of lifesaving devices and fluids. And, when it comes to consumer products, plastic gives us freshness and convenience. Our modern life depends on plastic.
“We fundamentally think plastics are a great way to package things like liquid laundry detergent,” says Martin Wolf, director of sustainability and authenticity at Seventh Generation, a company headquartered in Burlington, Vermont, USA, that specializes in responsibly sourced cleaning and personal care products. “What is needed is the will and the infrastructure to see that bottles are collected and reused or recycled.”
Most corporations, like Unilever, Starbucks, and PepsiCo, now have sustainability initiatives to ensure that the packaging used for their products no longer contributes to the problem of marine waste.
Sustainability goals
Founded on a mission of environmental sustainability, Seventh Generation continually evaluates their packaging to reach their most recent goals. “We think that any resource we use on this planet today needs to be available to future generations,” says Wolf. His company set a goal to make all their packaging material 100% recyclable by 2020.
Creating a recyclable bottle from post-consumer recycled (PCR) plastic is not easy. Each time plastic is recycled, its polymer chains are cut shorter, weakening the resulting polymer’s structure and limiting its reuse to one or two cycles. Bottle designs for recycled plastics must be carefully considered to ensure stiffness and avoid faults that may cause cracks under environmental stress. Wolf says it helps for the bottle blower and the resin supplier to have a good relationship. That way, the bottle blower can determine the quality of the resin and communicate directly with the supplier if changes need to be made.
Even when a bottle’s design is sound, packaging tricks may be needed to distract consumers from cosmetic imperfections, such as the grey tinge of a PCR bottle. Since Seventh Generation does not use dyes in their detergents, this slight coloration is noticeable. Adding a colored label to the bottle makes the greyness less obvious and more appealing to consumers who expect a cleaning product to look clean.
For all the work the company puts into their bottles, Wolf acknowledges that consumers are ultimately responsible for getting them to a recycling facility. If their municipality has no facility, the bottle goes into a landfill. Instead of waiting for local governments to establish recycling infrastructure, many companies provide the resources to expedite the expansion or construction of recycling programs around the world.
Henkel is a German chemical and consumer goods company headquartered in Düsseldorf, Germany. According to their website the company has dedicated itself to establishing a functioning waste and recycling management system for plastic materials to overcome the lack of a global standardized approach to handling waste responsibly. They are partnering with an organization called Plastic Bank, which provides people in impoverished communities with an incentive to recycle plastic waste by paying them for the plastic they collect. (https://www.plasticbank.com).
Henkel has also joined more than 250 large corporations including Nestle, Unilver, PepsiCo, and Walmart in a global initiative known as the New Plastics Economy (https://newplasticseconomy.org). The group is working with the United Nations (UN) Environment to establish a circular plastic economy and eliminate the current mode of using plastic once and then throwing it into a landfill. Participants recently signed a Global Commitment to hold each other accountable on a set of targets to improve plastic packaging management. These targets include a commitment to decreased plastic packaging and increased recycling and composting rates.
Though these efforts will go a long way toward reducing environmental plastic waste, Wolf says more needs to be done. “Recycling alone is not going to solve this problem, but it is one arrow in the quiver of solutions that people are going to have to develop,” he says. “There is going to have to be development of reusable packaging. There is going to have to be development of materials that somehow [like paper] are recycled in nature.”
A company like Seventh Generation that sells cleaning products has different packaging considerations than one selling products people eat or drink, such as food and beverages. In the United States, for example, the Food and Drug Administration has deemed a limited number of compounds safe for use as adhesives and coatings in food packaging (https://tinyurl.com/y92ystmw). To abide by these regulations and achieve sustainability, food manufacturers are seeking new sources for these compounds.
Natural solutions
David Grewell, manufacturing engineering professor at North Dakota State University, Fargo, and adjunct professor at Iowa State University, Ames, is also the director of the Center for Bioplastics and Biocomposites (CB2). The National Science Foundation-funded center consists of a team of university researchers based at Iowa State and Washington State University, in Pullman, who collaborate with automotive, petrochemical, and food packaging industries around the world to establish new plastic processes and products from renewable resources. The center will be expanding this year to include North Dakota State University, Fargo, and the University of Georgia, Athens, is considering becoming a member.
Grewell and the members of CB2 put nature to work solving problems in polymer science. “Nature has been making fibers for a long, long time; longer than we have,” he says. “She's designed these things to be very strong and lightweight. So, there are some advantages to these materials.”
Potted plant packaging is an example of one successful product resulting from the research at CB2. Researchers at Iowa State developed a plastic plant container made of polylactic acid (PLA) derived from a mix of corn and soybean proteins. The natural polymer has a fertilizing effect, because nitrogen is released from the soy protein as it decomposes. Instead of throwing the pot away, gardeners can crush it and place it in the transplant hole, where it will provide nutrients for the plant.
“The pots are degradable and they also contribute to the plant’s health. That was an effect we did not anticipate at all,” says Grewell. “So here is an example where nature has done something to those proteins with a cause and effect we did not anticipate. This would be very difficult to replicate with a petrochemical plastic.”
Another CB2 member, professor Tong Wang, has developed a soybean oil-based wax that can make coated paper packaging easier to recycle. The waxes are created by modifying the structure of hydrogenated soybean oil to behave like waxes derived from petrochemicals. These waxes could be used to coat corrugated paper boxes for packaging fruits and vegetables, or for the paper coating on milk cartons (https://tinyurl.com/yablmryv).
Consumers and packaging producers should be aware that the bio-based or biodegradable label is difficult to interpret. Naturally derived polymers do not necessarily produce less plastic waste. Bio-based monomers that are drop-in equivalents for petroleum monomers polymerize to the same non-biodegradable products. Just like plastics from petrochemical starting materials, their bio-based equivalents have a carbon backbone that does not readily decompose; although they can be recycled and used as PCR. Even plastics like PLA that are deemed compostable require waste facilities capable of heating polymers to temperatures higher than can be achieved in a backyard composite bin. Consequently, PLA plastics cannot just be thrown in a landfill with the expectation that they will convert back to soil in a reasonable amount of time.
Rather than leaving bio-plastics in the ground to decompose, countries like Denmark and Sweden have installed some power plants that burn plastic. “We put a lot of energy into making plastic, whether it is petrochemical or bio-based. I think that there are some advantages of recapturing that and using it as an energy source,” Grewell says. Energy conversion plants are also popping up across the United States, but they are not an option for a widespread plastic waste solution since the plants are too expensive for most municipalities. (https://tinyurl.com/ybt9tcq7).
To reach goals they have set for 2025, large companies need changes they can implement now. PepsiCo has committed to reducing the environmental impact of their packaging by investing in both bio-based plastics and new plastic sources. The company acknowledges that bio-based plastics reduce the greenhouse gas emissions that accompany petrochemical processing. But PepsiCo aims to have its entire packaging distribution incorporated into a circular plastics economy within the next six years, and that requires innovation.
New chemistry
In 2017, PepsiCo partnered with Danimer Scientific, a company dedicated to developing truly compostable plastic packaging. Using specially engineered bacteria that metabolize canola seed oil, Danimer Scientific produces a polyester called polyhydroxyalkanoate (PHA). In a recent study performed through a collaboration with the University of Georgia, Athens, the company found that after 85 days in soil and 148–195 days in saltwater, its PAH polymers biodegrade until they are indistinguishable from cellulose powder (https://doi.org/10.1021/acs.est.7b06688). In September of 2018, Danimer Scientific purchased a fermentation plant in Winchester, Kentucky, to begin full-scale PAH production.
While Danimer Scientific succeeded in using biology to synthesize polyesters, other researchers continue to explore the capabilities of chemistry. Rong Tong, assistant professor at Virginia Tech in Blacksburg, has designed a catalyst to polymerize a monomer that gives polyesters greater functionality.
Chemically derived polyesters most often start with lactide or β-lactones that are polymerized by a ring-opening. However, the stereoselective nature of this mechanism prohibits a diversity of side chains, thus limiting the resulting polymer’s properties. Tong has created a polyester from O-carboxyanhydrides (OCA) using a photocatalyst that allows him to control polymerization (https://doi.org/10.1038/s41467-018-03879-5).
Tong’s lab is the first to synthesize high-molecular-weight polymers using O-carboxyanhydride monomers. Tong says that in just two steps he can synthesize the monomer with a variety of different functional groups on its side-chain. This versatility motivated Tong to understand the chemistry needed to polymerize the monomer. He combined zinc and nickel to create a catalyst that is activated by light. “We hope this plastic can be a degradable and sustainable replacement for non-degradable plastic waste,” says Tong. His group is in the process of testing the properties of these new plastics to determine how they can be applied to packaging.
Another chemist whose research could increase polyester’s packaging potential is Eugene Chen at Colorado State University in Fort Collins. He identified a way to recycle polyester back to its original monomer. Chen can collect the recycled monomer at a yield of 80–90%, then purify and use it for polymerization again (https://doi.org/10.1126/science.aar5498).
Increasing polyester’s functionality and recyclability could encourage packaging producers to consider the material for more applications. “Traditional, non-degradable polyolefins are more widely used in thermal impact or thermal sets or resins or rubbers,” says Tong. He explains that the polyesters he and Chen are developing must exhibit similar properties to those polyolefin products to be considered marketable. Current European market data indicate that bioplastics contribute just 1% of all plastics sold (https://www.european-bioplastics.org/market/). As new polyesters enter the market, Grewell predicts that number will increase. “You are going to see more and more acceptance as more and more PLA comes online,” he says.
Meanwhile, petrochemical plastics production in the United States is experiencing a significant increase to accommodate demands in India and China. Billions of dollars have been invested to build and expand manufacturing plants along the US Gulf Coast. Wolf says the petrochemical industry missed an opportunity by only focusing on production. If they had embraced recycling early on, he explains, they could have developed the infrastructure to reprocess recycled plastic while still selling virgin resin. “There will always be a demand for product, but because they didn't (and still don't) want to support actual recycling, they are excluding themselves from that business opportunity.”
There is evidence that the petrochemical industry has decided to correct that oversight. In October 2018, Dow Chemical created two new positions that, according to a press release, identify ways the company can monetize plastic waste recycling streams in North and South America.
An alliance of 30 investors have recently compiled $1 trillion dollars to fund projects that contribute to a circular plastics economy or the development of recycling facilities (https://tinyurl.com/y8opuut9). Time will tell if investment dollars and public pressure can force the lifestyle shift necessary to eliminate the buildup of plastic waste, but any solution to the problem will have to overcome rising recycling costs and our ongoing dependence on plastic.
Rebecca Guenard is the associate editor of INFORM at AOCS. She can be contacted at rebecca.guenard@aocs.org.
References
- Biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) plastic under anaerobic sludge and aerobic seawater conditions: gas evolution and microbial diversity, Wang, S., et al., Environ.Sci.Technol. 52: 5700–5709, 2018.
- Stereoselective photoredox ring-opening polymerization of O-carboxyanhydrides, Feng, Q., et al., Nat. Commun. 9: 1559, 2018.
- A synthetic polymer system with repeatable chemical recyclability, Zhu, J.-Bo, E. Watson, J. Tang, and E. Y.-X. Chen, Science 3687: 398–403, 2018.
No more microbeads. Now what?
Where have cosmetics formulators turned now that the days of microbeads are officially over? As of July 1, 2018, companies in the United States can no longer manufacture rinse-off cosmetics containing microbeads. And beginning July 1, 2019, it is illegal for a company to sell any microbead products remaining in inventory (https://www.congress.gov/bill/114th-congress/house-bill/1321/text). The United Kingdom passed a ban the following year which took effect in January of 2018. New Zealand’s ban will be enforced starting in June, and in November 2018, an industry insider told Chemical Watch that it is highly likely that China will follow the global trend to impose a ban.
Legislation to ban microbeads — defined as, “any solid plastic particle that is less than five millimeters in size and is intended to be used to exfoliate or cleanse the human body or any part thereof”— passed swiftly and unanimously in the United States in 2015 after tens of millions were skimmed from the surface of the Great Lakes (https://doi.org/10.1016/j.marpolbul.2013.10.007).
Plastic microbeads are made with polyethylene, which is not toxic to humans, but polychlorinated biphenyls (PCBs) and other harmful environmental contaminants that are primarily hydrophobic concentrate on the large surface area of microbeads. Fish eat microbeads, because they look like food. Humans can then be exposed to these concentrated toxins when they eat fish (https://doi.org/10.1021/acs.est.5b06280).
The beads were so small that they evaded filtration during treatment at municipal plants and ended up in waterways. Attempts to filter the beads from open water also removed lower food chain species like plankton that were essential for the ecosystem’s survival. The only option was a complete ban, but where does that leave the cosmetics industry?
Several natural alternatives to plastic microbeads exist, but using them requires that formulators consider a few issues. Natural ingredients can degrade quickly, dissolving into a product, altering its exfoliating effect. And natural alternatives can induce allergic reactions in certain segments of the population. Nutshell powders, like almond and walnut or ground shellfish hold potential as inert abrasives in formulations, but not if a consumer breaks out in a rash when using them.
Fortunately, companies can choose from a long list of other ingredients. Some current exfoliants on the market contain pumice, silica, and apricot kernels. Others use sugar, sea salt, cellulose and castor oil. In every case, the exfoliating particles must be ground to between 125–250 micrometers to achieve the exfoliating affect without being too abrasive, especially for applications on the face where skin is thinner.
Sugar and sea salt exfoliants meet size variability requirements but have the disadvantage of being water soluble. While this property is environmentally favorable, achieving a stable shelf-life requires anhydrous or supersaturated formulations. Consumers will then experience less abrasiveness as the exfoliant dissolves with use. On the other hand, water insoluble abrasives made from biodegradable materials like cellulose fibers derived from plants and wood cannot be processed into spheres, which limits their applications.
Jojoba exfoliators, waxy spheres made from a liquid wax ester that results from pressing or extracting the oil of the jojoba seed, are another alternative. Their properties are relatively easy to control through hydrogenation. In fact, Jojoba is so similar to polyethylene that it is often mistaken for the banned microbead. See Table 1 for a comparison of polyethelene microbeads vs. replacement options.
|
TABLE 1. Comparison of polyethylene beads vs. replacement options. Reprinted with permission From Cosmetics & Toiletries, November 17,2017 |
|||||||||
| INCI | Density (g/mL) | Particle size range (diameter, in micrometers) |
Melt point °C | Relative cost | Abrasiveness | ||||
| Polyethylene a | 0.95 | 200–400 | 125–135 | $ | Moderate | ||||
| Polyethylene b | 0.95 | 50–200 | 125–135 | $ | Moderate | ||||
| Polyethylene c | 0.92 | 50–297 | 125–135 | $ | Low | ||||
| Polyethylene d | 0.92 | 50–180 | 125–135 | $ | High | ||||
| Polyethylene e | 0.92 | 44–150 | 125–135 | $ | High | ||||
| Jojoba esters f | 0.905 | 250–425 | 66–70 | $$$ | Low/Moderate | ||||
| Hydrogenated castor oil g; hydgrogenated jojoba oil | 0.953 | 150–250 | 82–90 | $$$ | Low/Moderate | ||||
| Stearyl Stearate (and) Helianthus anus (sunflower) seed wax and jojoba esters h | 0.959-0.967 | 250–425 | 66–72 | $$$ | Moderate | ||||
| Behenyl behenate (and) jojoba esters I | 0.928-0.936 | 425–850 | 69–75 | $$$ | Moderate | ||||
| Cellulose j | 0.45 | 200–600 | n/a | $$ | |||||
| Cellulose microcrystalline Cellulose k | 0.75 | 200–500 | n/a | $$ | Moderate | ||||
Finally, there are the truly innovative beads that are not only biodegradable, but also absorb unhealthy chemicals (https://doi.org/10.1007/s41742-018-0066-2). Scientists in Puerto Rico have designed a bead that pulls the coral bleaching compound oxybenzone, a UV-blocking ingredient in sunscreen, from ocean waters (https://www.popsci.com/microbeads-safe-environment). A formulator could potentially find a way to incorporate the beads into soaps and gels providing a benefit to both the consumer and the environment. The ban on microbeads has challenged formulators to explore new ingredients, but it has not reduced the availability of exfoliation ingredients and has already led to new innovations in the field.
References
- Microplastic pollution in the surface waters of the Laurentian Great Lakes, Eriksen, M., et al., Marine Pollution Bulletin 77: 177-182, 2013.
- Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish, Wardrop, P., et al., Environ. Sci. Technol. 50: 4037–4044, 2016.
- Simultaneous adsorption of cationic and anionic dyes by chitosan/cellulose beads for wastewaters treatment, Vega-Negron, A.L., L. Alamo-Nole, and O. Perales-Perez, et al., Int. J. Environ. Res. 12: 59–65, 2018.
