Do oil color scales make you see red . . . or yellow?
By Eric Umbreit and Matthew Russell
November/December 2013
- Color measurement is a critical aspect of oil quality.
- Various scales for the measurement of edible oil color have been created by industry and have evolved. Unfortunately, apparent similarities between these color scales can mask critical differences, sowing a degree of confusion among decision makers.
- Understanding the evolution and differences between these scales will aid in selecting and communicating the correct color scale, eliminating confusion and costly misunderstandings.
Among the many tests that need to be carried out on edible oils and fats during the refining process is the measurement of color. Color measurements are used not only to ascertain aesthetic quality but also as a means to optimize bleaching, deodorizing, and other production processes. Most, if not all, refined oils are sold on the basis of their color, and each type of oil will have its own “sell by color” instructions. So, it is necessary to monitor each stage of the refining process to establish whether the correct color has been reached.
Some crude seed oils can have unexpectedly high pigmentation, often attributable to adverse growing conditions, such as too little or too much moisture or frost damage to the plant (Meloy, 1953). As a result the color tends to darken in storage. Early color measurement often can alert the refiner to potential expensive bleaching and blending problems. Mixing problem oil with “in spec” oils can compound the situation as the darkening effect is carried over(Fash, 1934). Obtaining color data regularly on oil suspected to be unstable would indicate its condition and help to avoid making incorrect decisions regarding blending.
There are many other reasons why color measurement of oils and fats is important, but ultimately it all relates to the cost of refining, the quality of the finished product, and what the product looks like to the end user. That end user may be a food producer who is very aware of how the color of the oil could enhance or diminish that product’s appearance.
An end user may not consciously notice the color of a cooking oil unless it appears different than usual; then, suddenly, color is all important. As soon as a color difference is perceived, the end user may infer that “different” means “not as good.” Consequently, it is the goal of the edible oil plant production and quality processes to produce a consistent product in color—from plant to plant, lot to lot, and year to year.
Color measurement methods and color scale confusion
Color is a perceptual property in human beings. Color derives from the spectrum of light (distribution of light energy vs. wavelength) interacting in the eye with light-sensitive cells. In the human environment, materials are colored depending on the wavelengths of light they reflect or transmit. The visible color spectrum runs from red through to blue wavelengths, approximately 360–720 nm.
Three things are necessary to perceive color: (i) a light source, (ii) an object, and (iii) an observer/processor.
Colors are broadly described by descriptive words such as white, red, yellow, green, light, dark, bright, dull, and the like. However, each person describes and therefore defines an object’s color differently.
As a result, objectively communicating a particular color to someone without some type of physical standard is difficult. Describing in words the precise color difference between two objects is very challenging.
A person’s perceptions and interpretations of color and color comparisons are highly subjective. Fatigue, age, gender, and other physiological factors can influence color perception.
But even without such physical considerations, each observer interprets color based on their personal perspective, feelings, beliefs, and desires. For example, some people may convince themselves that a certain color match is within tolerance if they are under pressure to declare a color match as acceptable.
To quote the great 19th-century British scholar Lord Kelvin: “When you can measure what you are speaking about and express it in numbers you know something about it; but when you cannot express it in numbers your knowledge is of a meager and unsatisfactory kind.”
With this in mind, over the years various methods have been developed to measure the color of edible oils and fats. Before the development of electronic instruments using multiple wavelengths and photodetectors, quantitative color measurements were completed by comparing the sample to known glass color standards.
One of these was the Tintometer® colorimeter, invented by Joseph Lovibond in England in the late 19th century. Lovibond was a brewer by trade—and he needed a method to consistently evaluate and regulate the color of his beer. The Tintometer uses a series of gradient red-, yellow-, blue-, and neutral-colored glasses. It is arranged with two adjacent fields of view, seen through the viewing tube, so that the product in the sample field and a white reflective surface in the comparison field are observed side by side, suitably illuminated. This is known as the Lovibond® color scale (AOCS Method Cc 13e-92.)
Lovibond’s original Tintometer instrument concept is still in use around the world—and currently marketed as the Lovibond Tintometer Model F colorimeter (Fig. 1). This instrument uses 84 glass standards [in incrementally higher color intensity of Red (R), Yellow (Y), Blue (B), and Neutral (N) colors] to match the sample color visually. The Lovibond Tintometer colorimeter and its color standards quickly became a reliable tool for measuring and communicating the color description of many products including oils and fats. Small differences in colors can be measured and expressed in an easily understood and quantifiable way.
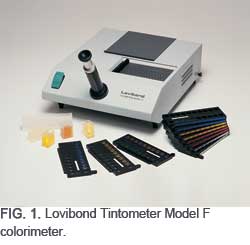
Early Tintometer instruments were supplied complete with a separate box of loose glass color standards each standard measuring 2ʺ× ¾ʺ (5 cm × 2 cm). The red, yellow, and blue colors of the standards are achieved by adding metallic oxides to each batch before committing to the furnace. Different shades of light to dark colors are obtained by varying the amount of metallic oxides in each batch for permanency of color.
However, during World War II, the ability to supply precise glass color standards became tenuous owing to war efforts. To respond to demand, Lovibond distribution partners in the United States cut the 5 cm × 2 cm loose glass standards into thirds, not realizing that the color matching for the Lovibond standards was completed in the center of the lower third of each glass standard. The upper part was intended for labeling and handling and did not require calibration. Unfortunately, over time, this action caused a skewing of the original Lovibond color scale, resulting in market confusion. Simply stated, instruments would not read the same.
In 1958, to resolve the discrepancies that had resulted from dividing the glass color standards, AOCS and US Bureau of Standards (now called the National Institute of Standards and Technology) worked with Tintometer Ltd. to resolve the issue. As a result, a new color scale, a homogenization of the Lovibond glass color standards, was created. This new color scale was measured using the term N" color units.
The Journal of the American Oil Chemists Society [39(3):25, 1962] announced in 1962 that the new scale was finally finished and approved. It was named the “AOCS-Tintometer Scale” (or “Wesson Method,” AOCS Method Cc 13b-45). The terms Lovibond and N" would be superseded by the AOCS-Tintometer scale in all results where compliance to Method Cc 13b-45 was required. Fundamentally, it is a modification of the Lovibond method—and focuses on the red and yellow colors—with no blue or neutral colors. With this instrument (called the Tintometer AF-710), it is possible to achieve a color match using only the red and yellow combination of standards. The lack of blue standards makes it necessary to ignore any difference in brightness and greenness. The visual-based instrument uses a physical gap between the sample and standard to account for brightness, unlike the Model F, which uses a neutral filter to make it duller. To this day, the AOCS-Tintometer scale is the most common color measurement scale used in the North American market.
Consequently, it should be pointed out that the red measurements of the Lovibond RYBN scale do not match those of the AOCS-Tintometer RY method. This creates confusion in the marketplace, as there is a tendency to report the “R” (red) value and not specify the color scale being used; 0.5 R on the Lovibond scale is not the same as 0.5 R on the AOCS-Tintometer scale. Much confusion can be averted merely by specifying/confirming the correct color measurement scale.
Beyond the aforementioned Lovibond and AOCS-Tintometer scales, various color scale offshoots have evolved, such as the AF960 Lovibond scale, which is an abridged red and yellow Lovibond scale that was introduced on an early electronic colorimeter that had a “shortened” measurement range of 0–20 Red with the same 0–70 Yellow color range.
Another Lovibond RY color scale variant is BS684, which is a modification of the Lovibond Scale. Technically, BS684 is not a scale but a standard. The BS684 variant of the Lovibond scale is optimized for measuring animal and vegetable fats by adding colorless glass compensating slides in the sample field as well as utilizing a black sheath to stop the entrance of any external ambient light from the sides of the cell. The racks containing the red, yellow, and blue color standards are fitted with clear, colorless glasses—known as compensating slides—in the lower row of holes so that they cover the sample field. The reason for the use of compensating slides is as follows: when light passes through a glass filter, a small percentage is lost at each of the glass surfaces owing to scattering and refraction, in addition to that which is lost through internal transmission due to the color of the glass. The result is a loss of brightness in the comparison field. To illustrate, the visual difference in color between 1.0 and 1.1 red is more noticeable than the difference between 0.8 and 0.9 red. The difference in saturation is similar between the two sets of filters, but the difference in brightness is down by about 8% for the higher-value filters due to the effect of light loss at the extra glass surfaces used to make up 1.1 red (a 1.0 red filter and a 0.1 red filter).
Compensating slides fitted in the racks counteract this brightness difference as they introduce the same number of glass surfaces into the sample field as are used in the comparison field to achieve a color match.
It should also be noted that, due to the high stability of glass standards, many instruments in the field are often of a venerable age. Unfortunately, over the decades they have often been modified and in many cases provide results that have drifted far from the standards. It is advisable such units be checked for compliance with calibrated glass or liquid standards.

Development of electronic color measurement instruments
As technology advanced, it became possible to measure color by using an electronic optical system, such as a colorimeter or spectrophotometer. However, this process was more challenging than would appear at first review. For instance, due to the vagaries of the human eye, how does one get an electronic photodetector to match the reading that most observers would see if they used the glass standards? Since each person sees color differently, in effect, the instrument would have to “average” the values that a range of observers would report for a specific sample with a particular color scale. This task was eventually completed by generating a massive amount of absorbance data for each of the color standards using the full spectrum of color wavelengths.
Regardless of whether an eye-based glass standard method or a modern electronic instrument is used, it is critical to specify which color scale is being measured (i.e., Lovibond, AOCS Tintometer, etc., including method reference), how samples have been prepared, and cell path length.
Eric Umbreit has been in the scientific instrumentation industry for over 15 years. He has a bachelor’s degree from Colorado State University (Ft. Collins, USA) and a master’s from Santa Clara University (California, USA). His email address is e.umbreit@tintometer.com.
Matthew Russell has over 15 years’ experience in the field of color measurement having worked for GretagMacbeth, X-Rite, and Lovibond Tintometer. He has an honors degree in chemistry from the University of Manchester and a master’s degree from the University of Manchester Institute of Science and Technology in the United Kingdom. His email address is m.russell@tintometer.com
Sidebar
How to reduce color communication problems
When comparing visual (subjective) to automatic (nonsubjective) color assessment, the fundamental differences between these methods need to be considered. Here are some basic steps to take to reduce color communication problems:
Make sure that you have a systematic, consistent, and reliable means of sample preparation and presentation
For example, when measuring liquids, are you using comparable, clean cells? Figure 2A shows the same liquid sample viewed across a range of cell path lengths. Figure 2B shows that, as path length changes, the perceived color of the samples will change significantly. Any visual or automatic methods’ results would be influenced by this difference.

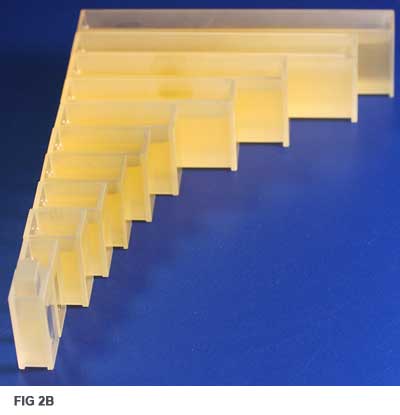
For example, with Lovibond RYBN Color, it is advisable that the depth of color never be greater than that which may be matched by a total of 20 Lovibond units.
The choice of pathlength will impact accuracy. Unless working to a particular specification, the optical path length of the cell used should be related to the color intensity of the sample—in a nutshell, the more intense the color, the shorter the pathlength.
When comparing with others, it is necessary to check that cell pathlength and type (optical glass, borosilicate, or plastic) are identical and the cells used are clean and undamaged.
Confirm that the correct color scale is selected on the automatic instrument
As discussed, historically a number of scales are available that report red and yellow values. This is a common source of error. For example; a standard Model F reports Lovibond red, yellow, blue, and neutral units (RYBN).
Information
- Meloy, G.W., Cotton and Cotton Oil Press 44(23):14 (1953).
- Fash, R.H., The effect of crude bollie cottonseed oil upon the color of refined oil from stored crude cottonseed oil, Oil and Soap 11:106–109 (1934).
- Zschau, W., Problems in bleaching differ with type of fat, Inform 1:638–644 (1990).
- Belbin, A.A., Color in oils, Inform 4:648–654 (1993).
