Separation anxiety: membrane cleaning in the 21st century
By Catherine Watkins
October 2015
- Membrane separation technology is a global industry that is expected to grow 9.2% annually through 2017, according to The Freedonia Group market research firm.
- The market for membrane cleaners formulated for specific types of foulants is also expected to grow.
- This represents a largely unexplored business opportunity for surfactant researchers, formulators, and manufacturers.
Right now, millions of industrial membranes are hard at work. They are separating macromolecules from seawater and wastewater. They are filtering out bacteria, concentrating proteins, and reclaiming process water in food production. They are removing particulates from flue gases in the petrochemical industry and accomplishing specific fractionations of high-value products in the biotech and pharmaceutical industries. In the process, they are all getting dirty.
No matter the type of filtration being conducted-whether reverse osmosis, ultra-, nano-, or microfiltration-each of these millions of membranes is becoming fouled and losing efficiency. Small membrane assemblies, known as elements, generally are discarded once they are fouled, but it is more cost-effective to clean larger assemblies. Many have to be cleaned frequently, some every day, most often in place. This, as it turns out, represents a huge business opportunity for surfactant researchers, formulators, and manufacturers.
Market background
Membrane technology is a comparatively recent development. Created in the 1960s when Sidney Loeb and Srinivasa Sourirajan synthesized the first asymmetric membranes, the technology took off in the 1970s and 1980s. Currently, about three-quarters of commercially available membranes are made of organic polymers; the rest are made of ceramic, metallic, or other inorganic materials, according to consultant H.S. Muralidhara of INSEPPPCON, LLC in Plymouth, Minnesota, USA. Muralidhara, formerly with Cargill, is a co-editor (with Z.F. Cui) of Membrane Technology (http://dx.doi.org/10.1016/B978-1-85617-632-3.00002-1, 2010, Elsevier Ltd.) and a frequent presenter on the topic.
Despite being new to the scene, membrane technology is a large and robustly growing field. Market research conducted by The Freedonia Group in Cleveland, Ohio, USA, found that global demand for membranes likely will increase by a healthy 9.2% annually to $25.7 billion in 2017. Rising environmental standards and regulations in many parts of the world as well as high population growth-particularly in water-stressed areas-are driving investment in membrane-based water and wastewater treatment systems. Increased attention to food and beverage safety regulations and rising interest in water reuse and material reclamation are also propelling membrane sales, Freedonia says.
The countries expected to see the fastest growth include the BRIC (Brazil, Russia, India, and China) countries and others with large, developing industrial bases and stressed local water resources. Combined, the United States and China, the two largest national markets for membranes in 2012, are expected to account for 43% of the market gains between 2012 and 2017.
A membrane separation primer
Membrane separation-which is a purely physical process and, therefore, uses less energy than traditional thermal separation processes that depend on a change in state-varies depending on the size of the separated particles and the separation mechanisms.
Industrial membranes are either porous or nonporous. Porous membranes are either microporous or asymmetric; they separate components in a mixture with pressure as the driving force. Nonporous (or dense) membranes generally are used to separate gases from gas mixtures. In this case, the membrane layer attracts molecules with high affinity, which are then diffused through the membrane, whereas molecules with low affinity are retained. An important caveat: This article deals mainly with the cleaning of membranes fouled via pressure-driven membrane processes operating on liquid feed streams.
The four primary pressure-driven membrane separation processes are classified by the pore size of the membranes and the transmembrane pressure (TMP) required. They are:
- Microfiltration (MF)-0.1-5 μm, 1-10 bar
- Ultrafiltration (UF)-500-100,000 Daltons (Da), 1-100 nm, 1-10 bar
- Nanofiltration (NF)-100-500 Da, 0.5-10 nm, 10-30 bar
- Reverse osmosis (RO)-<0.5 nm, 35-100 bar
The RO process is widely used for desalination and wastewater treatment; the UF and MF processes are commonly used in food, bioprocessing, wastewater processing, and industrial separation processes.
Organic membranes generally are manufactured from various polymers, including cellulose acetate, polyamide, polysulfone, polyethersulfone, polyvinylidene fluoride, polypropylene, and the like. Inorganic membranes-produced from ceramic, metallic, or other inorganic materials-are mechanically strong and chemically and thermally stable. They also tolerate a wider variety of temperatures, pHs, and pressures. However, they are also much more expensive than polymeric membranes.
Industrial membranes are assembled within devices and hardware, often called modules or elements (see Fig. 1), in order to separate the feed stream into retentate and permeate streams. The four main types of modules are tubular, hollow fiber, flat sheet, and spiral-wound.

Fig. 1. Membrane assemblies, known as elements, generally are thrown out once they are fouled, but it is more cost-effective to clean larger assemblies. Photo courtesy of Avista Technologies, Inc.
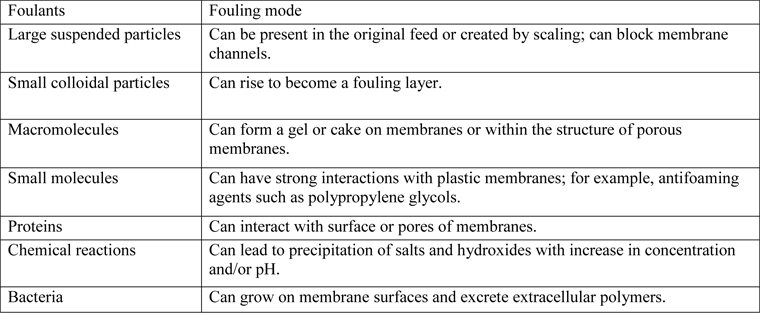
Suspended or dissolved substances deposited on the surface of industrial membranes-known as foulants-result in performance losses from a reduction in the permeability (or flux) of the membrane (see Table 1). Factors that influence the rate of fouling include:
- The nature and concentration of solutes and solvents,
- The type of membrane,
- The pore size distribution,
- The surface characteristics and material of the membrane, and
- The hydrodynamics of the membrane module.
Membrane cleaning basics
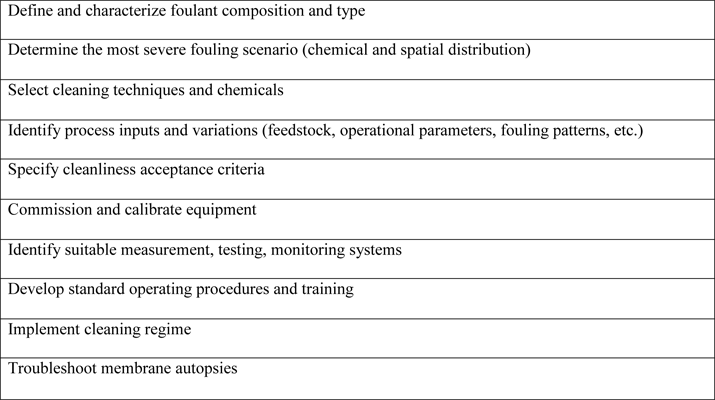
Cleaning time, temperature, hydrodynamic conditions, and concentration of cleaning agents all affect membrane-cleaning efficiency, as Hongyu Li and Vicki Chen point out in Membrane Technology (http://dx.doi.org/10.1016/B978-1-85617-632-3.00002-1). In addition, cleaning protocols must be adapted for each application and location. Table 2 summarizes the steps used in developing a cleaning regimen.
Membrane cleaning can be costly. Consultant Gerold Luss of Luss Consulting in Minneapolis, Minnesota, USA, notes that the annual cost of cleaning a single membrane unit in a dairy plant-which, of course, varies with size-can be as much as $30,000 for a small unit to $150,000 for a large unit. Membrane cleaning costs for seawater desalination also vary widely, Luss says, ranging from $5.00 per cleaning for a small undersink unit to $50,000 per cleaning for a large plant that provides water to a major city.
"Membrane chemical cleaning formulations are some of the more expensive formulations because they are tailored to the specifications of the membrane, the application for which the membrane is used, and also the expected cleaning conditions," says Luss. "That being said, I would place membrane cleaning chemicals at about the 75th to 80th percentile in terms of cost in industrial applications."
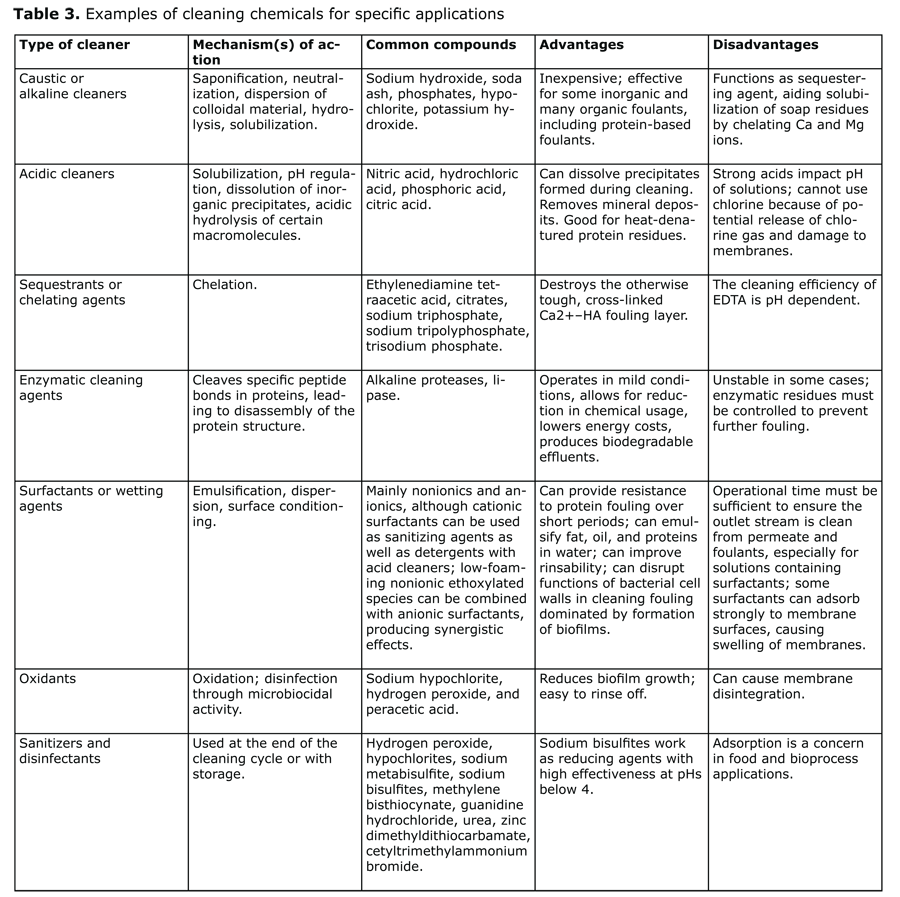
Most membrane cleaning is done in place, generally when the flux rate through the membrane decreases by 10% and/or the TMP increases by 10% and/or the permeate water quality decreases by 10%. The typical clean-in-place (CIP) sequence for membrane systems includes a pre-rinse, detergent wash, post-rinse, acidified wash, a second alkaline wash supplemented with chlorine when the membrane is compatible with oxidizing agents, and a final rinse. The mechanisms involved include dissolution, chelation, oxidation, hydrolysis, and emulsification (see Table 3).
 Sources: Membrane Technology, Ch. 10, Membrane fouling and cleaning in food and bioprocessing, Hongyu Li and Vicki Chen, Elsevier Ltd., 2010;Xiafu Shi et al., Fouling and cleaning of ultrafiltration membranes: a review, Journal of Water Process Engineering, 2014.
Sources: Membrane Technology, Ch. 10, Membrane fouling and cleaning in food and bioprocessing, Hongyu Li and Vicki Chen, Elsevier Ltd., 2010;Xiafu Shi et al., Fouling and cleaning of ultrafiltration membranes: a review, Journal of Water Process Engineering, 2014.
Felicity Plansky, a membrane technical field specialist at Hydrite Chemical Co. in Brookfield, Wisconsin, USA, offered a handy mnemonic device for remembering which surfactants work well in membrane cleaning formulations during a workshop at Cornell University in March 2014. In brief: cationic = catastrophic (because they are not compatible with negatively charged membranes), nonionic = nonproblematic, and anionic = applicable. Plansky also noted that membrane manufacturers issue very strict limits on the temperatures allowed for cleaning in place for many membranes. Typically, for UF and MF membranes, the temperature must be below 130°F, and for RO and NF membranes, the temperature should be below 118°F. However, recent developments in polymeric membranes now allow CIP at high pH (12+) and higher temperatures (up to 180°F) for certain membranes.
How membrane cleaning has changed
Membrane cleaning has changed both quantitatively and qualitatively in the past 40 years, according to a number of industry observers.
For example, three membrane specialists from Sealed Air in Charlotte, North Carolina, USA, find that the biggest change has been in the use of more generic chemicals for cleaning membranes, especially for cleaning UF membranes. Dennis Schmidt, Flemming Skou, and Gaetano Redaelli-who submitted written comments for this article-also noted that even RO membranes are being cleaned more with basic acid and caustic than in the past. Schmidt is an account manager and Flemming is a senior applications fellow, both with Sealed Air Hygiene Solutions. Redaelli is vice president and global sector lead with Sealed Air Food & Beverage.
"When membrane technology first appeared, most cleaning was done with formulated cleaners," they wrote, adding that the development of membranes that can withstand higher cleaning temperature and have higher pH tolerances "has taken membrane cleaning into new processes that weren't possible before. For example, some membrane plants in gelatin production can now be cleaned without the use of active chlorine, yielding longer membrane life."
The first membranes were made of cellulose acetate, which fouled less than today's polymeric membranes, says Dan Comstock, a founder and director of R&D at Avista Technologies, Inc. in San Marcos, California, USA. Because of water shortages the world over, he added, feed waters for RO filtration are being used "that you would never have considered" in the past.
"The unit cost of generic cleaners is certainly less than formulated cleaners," notes Comstock, "but generic cleaners are generally not as effective. Therefore, an overall economic case can be made for formulated cleaners, for they can extend membrane element life and reduce energy costs." The proliferation of UF and MF membrane systems has increased the business opportunities for surfactant researchers and manufacturers, he adds, while noting the need for low-temperature cleaners as well as formulations that are compatible with hard water.
Comstock illustrates the analytical challenges of membrane fouling with an anecdote. "A reverse osmosis system owner thought he had a simple case of silica scaling," he says. "An autopsy showed the presence of silica, aluminum silicates, iron phosphate, and calcium phosphate. With the aid of Chromatic Elemental Imaging and a process called heterogeneous nucleation, we were able to deduce that the aluminum silicates, iron phosphate, and calcium phosphate were triggering silica precipitation. By changing the scale inhibitor to one that inhibits these sparingly soluble salts, the customer has been able to operate his system silica scale-free."
Scope of the opportunity
Although water is one of the most important elements in membrane cleaning-representing 99% of any membrane cleaning solution-surfactants, and particularly surfactants in tailored formulations, are critical to membrane cleaning.
"Surfactants are key and will be key in the future," says Manuel Soria, global product manager, membrane chemicals, with GE Water and Process Technologies in Barcelona, Spain. Soria agrees that RO and NF membranes are now used under more stress conditions because of worse-quality water containing more foulants. This leads to the need for more sophisticated formulations.
"Today, the materials that create difficulty with removal are mainly organic and biofoulants," he says. The GE labs receive membranes that have special problems, he notes. "In these cases, we found that 70-85% of the contaminated membranes are mainly fouled with organic material from bacteria. This is more difficult to remove. In order to keep the system working, you need to remove this material or you need to change the membrane, and surfactants are necessary for penetration of the cleaner into the membrane foulant."
Among the difficulties in formulating membrane cleaners is ensuring the stability of formulations, Soria says, particularly with regard to formulations that are stable as solids but not as liquids. Also, increasing the concentration of surfactants in the formulation brings with it the risk of foaming. "As more surfactants are required to clean difficult foulants, there is a greater need to develop low-foaming surfactants with minimal environmental impact," he adds.
Perhaps the greatest need, according to Comstock, is to "try to get rid of trial and error in cleaner formulation and membrane autopsies and inject science into the process.
"If I have any legacy," he adds, "I hope that will be it."
Catherine Watkins is an associate editor of Inform. She can be contacted at cwatkins@aocs.org.

