Good vibrations: online and at-line monitoring of edible oils with vibrational spectroscopy
July/August 2016
- Vibrational spectroscopy encompasses mid-infrared, near-infrared, and Raman spectroscopy.
- Online and at-line (portable) infrared spectrometers allow rapid, reliable monitoring of edible oils at all stages of production.
- In addition, infrared spectroscopy can be used to assess the quality and authenticity of edible oils and products that contain them.
In almost every industry, there is a drive toward smaller, smarter, more user-friendly instrumentation, and the edible oil industry is no exception. Moreover, edible oil producers want instruments that are less costly, more environmentally friendly, and capable of monitoring every step in the production process from oilseed or fruit to the finished product. Further down the supply chain, food manufacturers want to ensure that the edible oils they use meet specifications and are not rancid or adulterated. To help satisfy these demands, many researchers and companies have developed technologies based on vibrational spectroscopy that can continuously monitor edible oil parameters on the production line (online), as well as portable devices that enable workers to obtain a quick snapshot of oil characteristics at different points during production (at-line).
Vibrational varieties
There are three major types of vibrational spectroscopy: mid-infrared (MIR), near-infrared (NIR), and Raman spectroscopy. All measure the effect of electromagnetic radiation on vibrational states of molecules in a sample. IR spectroscopy uses light within the MIR (2.5–25 μm wavelengths) or NIR (0.7–2.5 μm) region to excite vibrations in molecules. The IR interacts with the molecules and causes their chemical bonds to vibrate. “Each functional group in a molecule, depending on the nature of the functional group and also on the molecules that surround it, is going to have different vibrations at different frequencies,” explains Luis Rodriguez-Saona, professor of food science and technology at Ohio State University, in Columbus, USA. “We are able to identify different compounds based on these unique vibrations.”
In contrast, Raman spectroscopy irradiates molecules in a sample with a laser of fixed wavelength, which causes molecules to vibrate and scatter the laser light. “Depending on the type of functional groups present in a sample, light is going to be scattered at different frequencies,” says Rodriguez-Saona. Because Raman is a confocal technique, any out-of-focus signal is eliminated. As a result, Raman can analyze samples through transparent packaging such as glass or plastic bottles or bags, eliminating the need to remove the sample from its container (Ellis, D. I., et al., http://doi.org/10.1039/c5ay02048d, 2015). Raman spectroscopy can also be combined with microscopy to identify and quantify chemical distribution within a sample.
Each technique has its own advantages and limitations. Typically, MIR spectra are easier to interpret because the MIR excites only fundamental stretching and bending vibrations of C-H, C-O, O-H, and N-H bonds, and the signals are usually more intense and distinct than those in NIR spectra. Higher-energy NIR can excite complex vibrations, known as overtones (doubly excited vibrational modes) and combinations (two or more vibrational modes occurring simultaneously). In NIR spectra, the overtone and combination bands can overlap, making interpretation more difficult. However, the combination bands in NIR spectra can provide more complex structural information than can be obtained from MIR spectra (Cozzolino, D., http://doi.org/10.1039/c5ay01792k, 2015). In addition, NIR can analyze samples through plastic bags.
NIR is more amenable to online analysis because conventional MIR is limited to very short path lengths of only a few micrometers. Path length refers to the distance light travels through a sample placed in a spectrometer. The requirement for a very short path length creates problems for viscous samples such as oils, which must be placed in tiny capillaries for analysis. The path length limitation of MIR can be partially overcome by the use of a technique called attenuated total reflectance (ATR). In ATR, the IR light is passed through a crystal that is in contact with the sample. The IR penetrates into the sample at a depth of only 3 μm or less, compared with 10–50 μm for conventional transmission MIR spectroscopy (Cozzolino, D., http://doi.org/10.1039/c5ay01792k, 2015). However, this method requires the oil to be placed on an ATR crystal for measurement, which, again, could hamper automated online analysis. Mettler Toledo (Griefensee, Switzerland) offers an online FTIR ATR system called the ReactIR 45P.
8220;Raman is a technology that is attracting more attention, especially in the food industry,” says Rodriguez-Saona. Although online Raman systems are more unusual, Kaiser Optical Systems (Ann Arbor, Michigan, USA) sells four versions of Raman analyzers for online process control, such as the RamanRxn4 analyzer. In the past, a limitation of Raman has been that the laser commonly used for the technique (a red laser, with wavelength of 750 nm) tends to excite fluorescence as well as scattering. “New systems are actually using a near-infrared laser, which is at 1064 or 1030 nm, that reduces the effect of fluorescence,” says Rodriguez-Saona. “Now, it also diminishes the signal, so here’s where the technology has advanced in getting detectors that are going to be able to detect very low levels of scattered photons. So we’re seeing more of the Raman with near-infrared lasers, which is something that is very exciting.”
Inside an IR spectrum
Of the three types of vibrational spectra, the MIR spectrum is the most straightforward to relate to specific functional groups in a molecule (Fig. 1). For an edible oil, the higher-frequency end of the spectrum (3,700–3,400 cm-1) corresponds to hydroxyl groups (e.g., water and hydroperoxides). If an edible oil sample is clean, dry, and mostly unoxidized, there is no appreciable MIR absorption in this region (van de Voort, F. R., and Sedman, J., Inform 11: 614–620, 2000). At lower frequencies (3,025–1,500 cm-1), different functional groups such as methylene, methyl, aldehyde, and ester groups absorb MIR at specific frequencies. At ~1,750 cm-1, a strong band arises from the ester linkage attaching fatty acids to the glycerol backbone of triglycerides. On the right shoulder of this band, a small band corresponding to the carboxyl group of free fatty acids can be observed, if lipolysis has occurred in the sample. In the same region, small carbonyl absorption bands of aldehydes and ketones are seen, if the oil is oxidized.
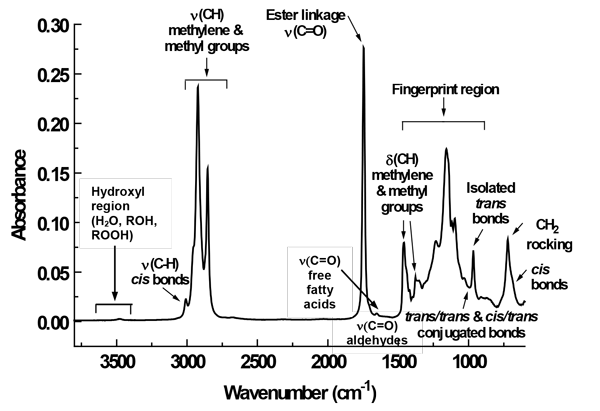
FIG. 1. The mid-IR spectrum of an edible oil collected on an attenuated total reflectance (ATR) crystal. Labels indicate absorption bands or regions associated with triglycerides or other constituents that may be present in an oil. FFA, free fatty acids. Inform 11: 614–620.
A region between 1,500 and 900 cm-1, called the “fingerprint region,” has a pattern of bands that is highly characteristic of molecular composition, and can therefore be used to identify substances. The fingerprint region contains structural information such as whether trans-containing triglycerides are present in the oil. If more than one double bond is present in a particular triglyceride, the fingerprint region can also indicate whether the double bonds are in trans/trans or cis/trans configurations.
Thus, an abundance of qualitative data, such as edible oil composition, moisture content, and oxidative status, can be gleaned from an MIR spectrum. In addition, quantitative data on oil composition can be obtained. According to the Beer-Lambert Law, the concentration of a molecule or functional group is proportional to its absorbance, or peak height. Therefore, vibrational spectroscopy can provide both qualitative and quantitative information about the components in an edible oil sample.
An evolving technology
IR spectroscopy has been used in the food industry for decades, with much of the fundamental work being carried out between 1945 and 1965 (van de Voort, F. R., et al., http://doi.org/10.1007/s12161-008-9031-6, 2008). In the early days, IR spectroscopy was used almost exclusively for qualitative purposes, mainly molecular characterization. An exception was the widespread use of MIR during the 1950s to determine trans isomers in edible oils and fats. Then, interest in IR spectroscopy lagged as newer techniques, such as gas chromatography (GC) and nuclear magnetic resonance (NMR) spectroscopy, became available.
In the 1970s, the advent of Fourier Transform (FT) IR spectroscopy sparked renewed interest in quantitative IR analysis. These instruments were a substantial improvement over the so-called dispersive IR instruments introduced in the 1940s. The old instruments scanned sequentially through wavenumbers of light. In contrast, FTIR instruments collect data from all wavenumbers simultaneously, and then translate the resulting interference pattern into an IR spectrum. FTIR instruments are faster, more sensitive, and more accurate than the older instruments. They have fewer moving parts prone to slippage, and allow no stray light. The digital processing capabilities of FTIR instruments enabled new chemometric techniques for qualitative and quantitative analysis. In the late 1980s, more compact, robust, and inexpensive FTIR instruments became available, which furthered the popularity of the technique (van de Voort, F. R., et al., http://doi.org/10.1007/s12161-008-9031-6, 2008).
Nowadays, “there is a trend toward usability and portability,” says Frederic Prulliere, business development manager at Agilent, in St. Louis, Missouri, USA. “People want instruments that are smaller, smarter, easier to use, and that can give you an answer right away. Space is often at a premium. With portable devices, you can bring the instrument where it is needed.” Agilent offers several portable and handheld FT-MIR instruments, such as the 4500 Series Portable FTIR (Fig. 2.). Prulliere notes that Agilent’s portable FTIR systems are very user-friendly. “You don’t need to have sophisticated, highly trained personnel, as you do with other techniques such as GC-MS.” Currently, Agilent does not offer FTIR instruments for online analysis. “Cost is a consideration,” says Prulliere. “An online system might cost $80,000 to $100,000, while a portable (at-line) system is only about $28,000.”

FIG.2. The Agilent 4500 portable FT-MIR instrument is rugged, compact, and easy to use, making it ideal for at-line measurements.Credit: Agilent
Online instruments on the market are typically based on FT-NIR. Bruker Optics (Billerica, Massachusetts, USA) offers the MATRIX-F FT-NIR spectrometer (Fig. 3), which can simultaneously measure multiple parameters of an oil at various stages of production or use. The instrument can be housed in a cabinet, away from hostile conditions on the factory floor, with fiber optic probes reaching difficult-to-access samples in tanks, bypasses, or conveyer belts. Up to six sensors can be multiplexed in a single MATRIX-F spectrometer. Other manufacturers such as Foss (Hillerød, Denmark), Unity Scientific (Brookfield, Connecticut, USA), and Thermo Scientific (Waltham, Massachusetts, USA) also offer at-line and online FT-NIR solutions.

FIG. 3. The Bruker MATRIX-F FT-NIR online system can remotely access hard-to-reach samples through fiber optics technology. Credit: Bruker
Quality control
At-line and online FT-NIR systems are finding applications in quality control and process monitoring during each step of edible oil production, from checking the incoming oilseeds to quality testing of the finished product (Bruker, http://tinyurl.com/BrukerFTNIR, 2015). FT-NIR spectrometers can non-destructively analyze both liquid and solid samples. When oilseeds arrive at a processing plant, FT-NIR can be used to check parameters like oil and moisture content, amount of free fatty acids, and fatty acid composition. The high-oleic status of specially bred oilseeds (e.g., high-oleic sunflower seeds) can be verified. Workers simply place ground or unground oilseeds in a cup with a glass bottom, and the FT-NIR spectrometer completes multiple analyses in less than one minute. In contrast, sending seeds to outside labs for analysis can take days and hold up production.
During oilseed storage, moisture analysis by FT-NIR can help monitor conditions and drying processes to discourage the growth of bacteria, fungi, and mold. To evaluate oil extraction efficiency, FT-NIR can monitor moisture and oil levels in seeds, expeller cakes, and extracts. The extracted crude oil can be analyzed for free fatty acids, phospholipids, and waxes in order to optimize refining conditions. And each step of the refining process can be monitored by online FT-NIR for real-time process measurements.
Furthermore, FT-NIR can be used to assess fat modification processes such as fractionation, interesterification, and hydrogenation. The technique can evaluate the physical and chemical properties of the finished oil, including fatty acid profile, free fatty acid content, the presence of trans fats, iodine value (IV, a measure of the amount of unsaturation), and solid fat content. And finally, FT-NIR can analyze oil byproducts (which can be used as raw materials for animal feed and other products) for oil, protein, moisture, fiber, and ash content.
Before FT-NIR and FT-MIR instruments became widely available, companies typically had to send samples to off-site labs, where edible oils were analyzed for quality parameters by wet-chemistry (e.g., titrations or chemical reactions) or chromatographic techniques. “A lot of it was based on just wet chemistry,” says Chris Dayton, director of fats and oils processing at Bunge Limited in White Plains, New York, USA. “And then we would have holdups in the system to qualify a tank of oil or protein before it was shipped. So it made for larger inventories and a higher risk of being out of specification.” Bunge, a global agribusiness and food company that manufactures edible oils and margarines, among many other products, uses both at-line and online IR systems for process monitoring, says Dayton. “You know instantaneously what’s going on so you can use statistical process control to run your operation,” he says. “You’re able to meet customer specifications with the least amount of cost involved.”
Traditional methods of analysis are usually designed to measure only one parameter, whereas FT-MIR and FT-NIR can analyze multiple parameters simultaneously. In addition, the IR methods are safer and more environmentally friendly than wet-chemistry or chromatographic methods because no hazardous chemicals or solvents are involved. In most cases, no sample preparation is required—the oilseed or oil is placed (either manually or automatically) directly into the instrument for analysis. Therefore, no special skills are needed, and there is little or no chance of operator error. Automated chemometrics (multivariate statistical analysis) packages in commercial systems can translate IR spectra into easy-to-understand qualitative or quantitative data. In the simplest terms, chemometric analysis involves comparing test IR spectra with stored spectra of known reference materials. For quantitative analyses, chemometric models can be developed that correlate FT-MIR and FT-NIR spectra with results from standard methods of analysis or with reference standards containing known amounts of analytes.
Examples of applications
Frying oils
In commercial frying operations, fats and oils are used continuously at high temperatures. During frying, hundreds of chemical reactions occur that change the physical and sensory properties of the oil and of the fried food. Oxidation of frying oils can cause off-flavors, foaming, and the formation of unhealthful compounds such as aldehydes and monomeric oxidized triglycerides (MonoxTG). Several wet-chemical and chromatographic methods have been developed to monitor oxidation products in frying oils. For example, AOCS Official Method Cd 18-90 uses a chemical reaction to determine anisidine value (AnV), a measure of aldehyde production. AOCS Method Cd 20-91 uses column chromatography to analyze total polar compounds (TPC) in frying fats. Although these methods are well validated, they are relatively slow, require a well-equipped lab and skilled staff, and necessitate the use (and subsequent disposal) of solvents and toxic chemicals.
To establish FT-NIR as a tool for monitoring frying oil quality, researchers recently conducted a large study of 400 fresh and used frying oils collected from restaurants, bakeries, industrial frying plants, fisheries, and other frying industries in Germany (Gertz, C., and Behmer, D., http://doi.org/10.1002/ejlt.201300270, 2014). The oils were of varied types and degrees of unsaturation, and were used to fry a variety of foods such as potato chips, battered fish, donuts, and chicken. The researchers used FT-NIR to simultaneously measure parameters such as AnV, TPC, MonoxTG, di- and polymerized triacylglycerols (DPTG), acid value (AV, a measure of free fatty acids), and fatty acid composition, including trans fats. They then analyzed the same samples using standard methods of the German Society of Fat Science (DGF), which were based on wet chemistry and chromatography. By comparing the FT-NIR spectra to the known values determined by standard methods, the researchers used a partial-least-squares (PLS) algorithm to construct calibration models. Then, they tested the models on a validation set of oils. The researchers found that all of the FT-NIR measurements correlated well with those determined by official standard methods. In the future, fast food restaurants or other frying operations could use online or at-line FT-NIR systems to decide when it is time to discard used frying oils.
In 2013, the German Society for Fat Science issued a standard method [DGF C VI 21a (13)] for using FT-NIR to determine DPTG, IV, AV, and AnV in used frying fats and oils (Gertz, C., et al., http://doi.org/10.1002/ejlt.201300221.)
Potato chip oils
Lipids comprise 35–44% of potato chips (Aykas, D. P. and Rodriguez-Saona, L. E., http://doi.org/10.1039/c5ay02387d, 2016). The most common frying oils for potato chips are corn, canola, sunflower (mid- and high-oleic), high-oleic safflower, and cottonseed. For economic purposes and to extend shelf life, many potato chip manufacturers use blends of oils, rather than a single vegetable oil. Edible oils are one of the most counterfeited foods, and they may be adulterated with cheaper oils such as canola, soybean, and palm. Potato chip manufacturers may unknowingly receive shipments of adulterated vegetable oils, which then end up in the potato chips.
To develop at-line methods for potato chip oil authentication, Rodriguez-Saona and his coworker used a portable FT-MIR spectrometer equipped with an ATR crystal (Cary 360, Agilent Technologies Inc., Santa Clara, California, USA) to analyze 95 commercial potato chips (http://doi.org/10.1039/c5ay02387d, 2016). The researchers expelled the oil from the chips with a hydraulic press. Then, they measured various oil quality parameters using both FT-MIR and standard techniques: for the fatty acid profile, gas chromatography-fatty acid methyl ester (GC-FAME) analysis; for peroxide value (PV), AOCS Official Method Cd 8-53; for free fatty acids, AOCS Method Ca 5a-40; and for AnV, AOCS Method Cd 18-90.
Using chemometrics [PLS and soft independent modeling of class analogy algorithm (SIMCA)], the researchers correlated the MIR spectra with the reference values obtained by the standard methods. They then tested the calibration model on 13 additional potato chip samples. The fatty acid profiles determined by portable FT-MIR correlated well with those obtained by GC-FAME. Eight of the potato chip samples contained only one type of oil, whereas five had mixtures. Three samples had mislabeled oils sources: The potato chip labels claimed only sunflower oils, but they also included cottonseed oil and at least one other oil. Using SIMCA, the researchers showed that different oil types formed distinct clusters (Fig. 4), providing a rapid screening tool for potato chip manufacturers.
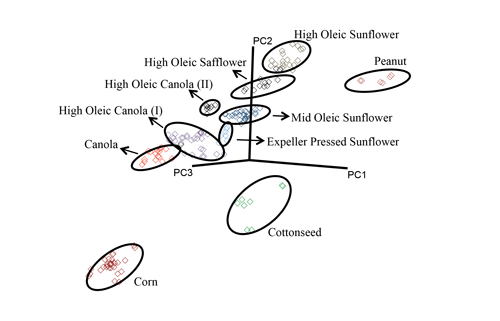
FIG. 4. (a) SIMCA 3D projection plots of second derivative-transformed spectral data collected by a portable FT-MIR spectrometer for frying oils extracted from commercial potato chips. Different oil types form distinct clusters. (b) SIMCA discriminating plot based on the MIR spectra of oils using a portable FT-MIR spectrometer, showing bands and regions responsible for class separation. Courtesy of Luis Rodriguez-Saona
In other work, Rodriguez-Saona and colleagues used a portable FT-MIR spectrometer and similar chemometric approaches to characterize the fatty acid compositions of dietary omega-3 oil supplements (Plans, M., et al., http://doi.org/10.1007/s11746-015-2666-8, 2015) and to differentiate conventional and organic bovine butters (Plans Pujolras, M., et al., http://doi.org/10.1007/s11746-015-2591-x, 2015).
Olive oil authentication
As a high-value oil, extra virgin olive oil (EVOO) is commonly adulterated with cheaper seed oils such as sunflower or hazelnut. Currently, no method is available to rapidly authenticate EVOO and identify and quantify adulterants (Azizian, H., et al., http://doi.org/10.1007/s11745-015-4038-4, 2015). Therefore, Hormoz Azizian, Magdi M. Mossoba, and colleagues used the MATRIX-F and –MPA FT-NIR spectrometers (Bruker Optics) to analyze EVOO, as well as EVOO spiked with known amounts of vegetable oil adulterants.
The researchers noticed that the FT-NIR spectrum of pure EVOO contains two characteristic minor bands near 5280 and 5180 cm-1, which were attributed to volatile and non-volatile components, respectively (Fig. 5). When the researchers spiked the EVOO with fully refined olive oil, palm oil, or other vegetable oils, the band near 5280 cm-1 team decreased in intensity. The researchers used these two bands and other spectral regions to develop PLS calibration models. Because the vegetable oil adulterants had different fatty acid profiles, the team had to develop four separate PLS models. When they analyzed a commercial sample with suspected adulteration, the researchers fit the FT-NIR data to one of the models, which predicted 11.7% adulteration with a high-linoleic-acid oil. The researchers confirmed this result by spiking a test sample with known amounts of soybean oil, which is high in linoleic acid.

FIG. 5. FT-NIR absorption spectrum of an extra virgin olive oil, showing the presence of weak yet highly characteristic bands at 5180 and 5280 cm-1. Credit: Lipids
Future prospects
Although vibrational spectroscopy has been available for decades, many food companies have yet to embrace the technology, partly due to familiarity with the old methods, and partly because of misconceptions that vibrational spectroscopy is too complex or expensive. “Based on my own experience, even when the benefits of a technology are as clear-cut as in the case of FTIR spectroscopy, overcoming the inertia of familiar standardized procedures can still be a daunting task, especially in the cost-conscious food industry,” says Frederik van de Voort, emeritus professor and co-director of the McGill IR Group at McGill University in Montreal, Canada.
However, recent advances in optics and chemometrics, as well as the availability of online and at-line FT-MIR and FT-NIR spectrometers, argue that the technology merits a second look. “It’s a very exciting time for this technology,” says Rodriguez-Saona. “It seems that the technology is more amenable now to what the industry needs.” In the not-too-distant future, portable vibrational spectrometers might even be placed in the hands of consumers, who could do their own quality checks of edible oils and foods at home or in supermarkets.
Laura Cassiday is an associate editor of INFORM at AOCS. She can be contacted at laura.cassiday@aocs.org.
Information
- Aykas, D. P. and Rodriguez-Saona, L. E. (2016) “Assessing potato chip oil quality using a portable infrared spectrometer combined with pattern recognition analysis.” Anal. Methods 8 : 731–741. http://doi.org/10.1039/c5ay02387d.
- Azizian, H., et al. (2015) “Novel, rapid identification and quantification of adulterants in extra virgin olive oil using near-infrared spectroscopy and chemometrics.” Lipids 50: 705–718. http://doi.org/10.1007/s11745-015-4038-4.
- Bruker. (2015) “Edible oils and fats: FT-NIR analyzers for QC in the lab and production.” Product brochure. http://tinyurl.com/BrukerFTNIR.
- Cozzolino, D. (2015) “The role of vibrational spectroscopy as a tool to assess economically motivated fraud and counterfeit issues in agricultural products and foods.” Anal. Methods 7: 9390–9400. http://doi.org/10.1039/c5ay01792k.
- Gertz, C. and Behmer, D. (2014) “Application of FT-NIR spectroscopy in assessment of used frying fats and oils.” Eur. J. Lipid Sci. Technol. 116: 756–762. http://doi.org/10.1002/ejlt.201300270.
- Gertz, C., Fiebig, H.-J., and Hancock, J. N. S. (2013) “FT-near infrared (NIR) spectroscopy–Screening analysis of used frying fats and oils for rapid determination of polar compounds, polymerized triacylglycerols, acid value, and anisidine value [DGF C-VI 21a (13)].” Eur. J. Lipid Sci. Technol. 115 : 1193–1197. http://doi.org/10.1002/ejlt.201300221.
- Ellis, D. I., et al. (2015) “Point-and-shoot: rapid quantitative detection methods for on-site food fraud analysis—moving out of the laboratory and into the food supply chain.” Anal. Methods 7 : 9401–9414. http://doi.org/10.1039/c5ay02048d.
- Plans, M., et al. (2015) “Application of infrared spectroscopy for characterization of dietary omega-3 oil supplements.” J. Am. Oil Chem. Soc. 92, 957–966. http://doi.org/10.1007/s11746-015-2666-8.
- Plans Pujolras, M., et al. (2015) “Portable infrared spectrometer to characterize and differentiate between organic and conventional bovine butter.” J. Am. Oil Chem. Soc., 92, 175–184. http://doi.org/10.1007/s11746-015-2591-x.
- van de Voort, F. R., et al. (2008) “Perspectives on quantitative mid-FTIR spectroscopy in relation to edible oil and lubricant analysis: evolution and integration of analytical methodologies.” Food Anal. Methods 1: 153–163. http://doi.org/10.1007/s12161-008-9031-6.
- van de Voort, F. R., and Sedman, J. (2000) “FTIR spectroscopy—The next generation of oil analysis methodologies?” Inform 11: 614–620.

