Alternative base oils: a perspective
By Raj Shah, Mathias Woydt, and Hillary Wong
March 2021
- Alternative base oils can improve fuel economy and performance as they can leave the engine oil cleaner for much longer compared to the traditional engine oil and reduce the material footprint.
- This increases vehicle mileage and allows the vehicle to reduce CO2 emissions without having to replace the engine.
- In the near future, it can be expected that there will be more lubricant formulas that incorporate alternative base oils.
More Informed Poster Summary
Download a PDF poster of this topic with additional figures and graphics.
This poster is part of a new digital platform from AOCS called More Informed. Visit the More Informed page to discover other opportunities to continue learning beyond the pages of the magazine.
Engine oil is a product that has existed for a very long time and dates back to as far as 1866, when the first branded engine oil emerged [1]. The technology of automobiles is always evolving; therefore, innovation of engine oils must keep up with the evolution of automobiles. Creating new formulas and compositions of engine oil is vital to allow the automobile to perform at its best with minimal wear and maximum protection to prolong engine life.
When creating new engine oil formulas, it is also essential to consider formulations that lower viscosity and improve friction properties. When viscosity is reduced and friction properties at boundary and mixed lubrication regimes are improved, the fuel economy, engine performance, and engine life can be improved. One notable aspect of engine oil formulas is the base oil as base oil is one of the fundamental ingredients that make up all engine oils. Base oil makes up about 75% to 80% of an engine oil, which is the majority of the product [2]. Figure 1 shows the percentages of the ingredients used to make engine oil.
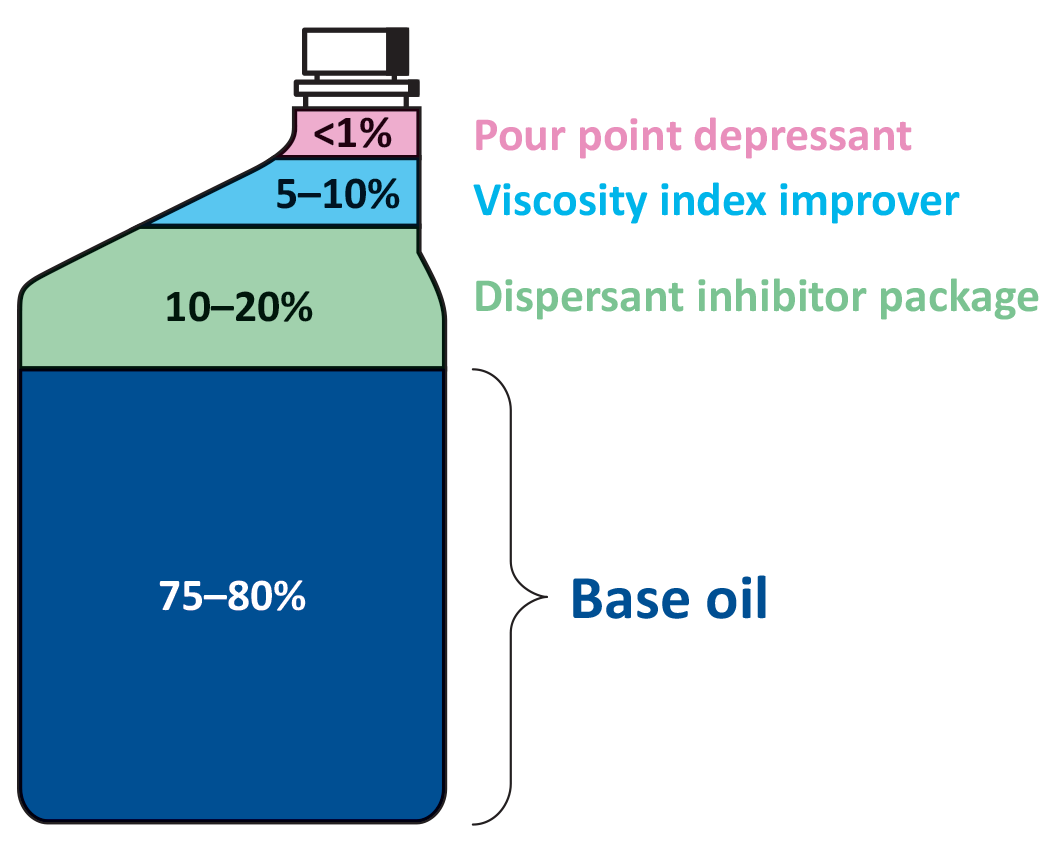
Base oil is typically produced by refining crude oil [2]. Base oil is categorized by API into five different groups, I–V. These groups distinguish the type of base oils by the way they are processed. Early traditional base oils are categorized in Group I and are produced by solvent-refining technology [4]. Group II and III are also refined from crude oil, except Group II has better antioxidation properties and is processed by hydrocracking (a two-step process that uses high pressure, heat, a catalyst, and hydrogen [5]), while group III is more refined and severely hydrocracked compared to group II. Group IV consists of polyalphaolefins (PAOs) and are processed by synthesizing. Group V consists of all other base oils such as esters, polyalkylene glycol (PAG), and bio-olefins. In Europe, polyinternal olefins (PIO) were added by ATIEL as a group VI base oil. Currently, Group II is the most common type of base oil used in engine oils.
Most current base oils are made from crude oil, a nonrenewable resource. Other alternatives for base oils are being researched and implemented into newer engine oil products. One of the alternative base oils is bio olefins in which many kinds of bio olefins are categorized in Group IV. Alternative base oils such as esters and PAGs are categorized as group V base oils. These alternative base oils have different characteristics with varying strengths and weaknesses. With different characteristics, each base oil will have specific and slightly different applications [6]. Table 1 compares the strengths and weaknesses of different base oils. The maximum operating temperature is an indication of thermal stability and depends upon the antioxidation additive packages used.
| Synthetic | Strengths | Weaknesses |
|---|---|---|
| Polyalphaolefins (PAOs)Maximum Operating Temperature 399°F/up to 204°C | High VI, high thermal oxidative stability, low volatility, good flow properties at low temperatures, nontoxic, compatible with mineral oils | Biodegradability depends from molar mass, limited additive solubility, seal shrinkage risk |
| Di-, tri-, tetraesters and Polyolesters Temperature 399°F /up to 204°C | Nontoxic and biodegradable, high VI, good low-temperature properties, miscible with hydrocarbons | Hydrolytic stability and miscibility with hydrocarbons can be an issue, limited seal and paint compatibility |
| Phosphate Esters Maximum Operating Temperature 241°F/116°C | Highest auto-ignition temperature, excellent wear resistance and scuffing protection | Moderate VI, limited seal compatibility, not miscible with hydrocarbons, moderate hydrolytic stability |
| Polyalkylene Glycols (PAGs)Temperature 399°F /up to 204°C | Low friction and excellent lubricity, nontoxic and biodegradable, high VI, good thermal and oxidative stability | Limitations in soluble additives, miscibility with other base oils depends from backbone, limited seal and paint compatibility |
| Silicones Maximum Operating Temperature 486°F/252°C | Highest VI, high chemical stability, excellent seal compatibility, very good thermal and oxidative stability | Not miscible with hydrocarbons and additives, weak lubricity under mixed/boundary lubrication |
Group V base oils stray from common market base oils and refining practices that are found in group I–III base oils. However, many Group V base oils are researched more in-depth as of late for the creation of new low-viscosity and friction engine oils. Alternative base oils may be needed, because the NOACK evaporation of low-viscosity hydrocarbons increases with decreasing viscosity. Esters and PAGs have much lower volatility due to their molecular polarity.
The Alternatives
Base oils other than the typical hydrocarbon base oils appeared in aircraft engines during WWII. The German synthetic lubricant base oils consisted of a blend of poly(ethylene)-ester concept, generated more or less no soot, and was a low-viscosity grade in the range of a 0W–20 engine oil of today. The kinematic viscosity at 100°C of SS1600 used in air cooled radial BMW 801 was ~6.2 mm²/s (SS= synthetischer Schmierstoff= synthetic lube). The ester was a reaction product of methyladipic acid with branched C8-C14 alcohols. The US Air Force used a polypropyleneglycolmonobutylether (Union Carbide Prestone 200, LB-550 base oil) from March 1944 onward with a viscosity at 100°C of 18.5 mm²/s (safer design!). The PAG based aircraft engine oil was ash-free and formed no deposits. Until today, the price level of such base oils limited the market penetration.
The key differences in properties between hydrocarbons to esters and polyalkylene glycols originate from the presence oxygen in their carbon backbones. Esters have one, two, or three ”ester” links with two oxygen atoms each, whereas PAGs have in each monomer an “ether” link. The oxygen represents an increase in molecular polarity, which increases the lubricity and viscosity index as well as reducing the NOACK evaporation. However, the polarity due to the oxygen atoms makes the compatibility with polymeric materials challenging. Esters are one of the Group V base oils added as a co-base stock for engine oils. There were many past attempts to market engine oils using 100% esters as a base oil stock. The use of esters in engine oil sparks interest in creating low viscosity, high viscosity index, and reduced friction engine oils that can improve fuel economy and reduce greenhouse gas emissions [7].
Esters are being implemented into low-viscosity engine oils due to their low volatility and high viscosity index (VI) characteristics. Esters further exhibit great thermal and oxidative stability properties which allow them to perform well at high temperatures [8] associated with low (reduced) NOACK volatilities (physical evaporation). Esters may go through a thermal degradation where oxygen is not involved [9]. This reaction where the ester may typically go through beta-elimination at temperatures of 275°C to 315°C, does not occur as metals act as a catalyst and lower the temperature to 200°C. Since there is “no hydrogen present in the beta position of oxygen” [9], beta-elimination is unlikely to occur and therefore, increases thermal stability. Esters also have great solvency, as they can solubilize complex additives into the formulation [10]. Due to esters’ high VI and great oxidative stability, the formulas of the engine oils that incorporate esters will use less VI improvers and dispersants, in which both VI improvers and dispersants are known to increase the viscosity of the finished engine oil product [7].
Esters can meet the criteria of environmentally acceptable lubricants (EALs) as per Vessel General Permit [10]. There are three different classes of esters that are commonly found in engine oils: diesters, triesters (trimethylolpropane esters (TMP)), tetraesters, and polyolesters. These esters can have great biodegradability and are miscible with other oils and additives [6]. Triesters are highly desired as they can be used in a wide range of viscosities. Esters have other properties that are suitable for engine oils. Esters induce a good seal swell in order to compensate for the shrinkage by PAOs and have low deposit formation which is good for preserving the lubricant for a long time without having to change the engine oil for a while [9]. Esters in engine oils also provide high flash points of up to 325°C. Having high flash points makes the engine oil safe as it is more resistant to ignition. An experimental engine oil was made with diesters (adipate ester or di-isotridecyl adipate) and polyolesters (TruVisTM P3020) to compare with engine oil using a Group II base oil [7]. Further testing showed that the engine oil with ester base oil had better depositing performance and friction reduction compared to the engine oil with Group II base oil. The results from the test indicates that esters used in engine oils provide low viscosity and low friction properties and therefore improve fuel economy and engine performance.
Another alternative base oil used to create low-viscosity and friction engine oil is PAGs. PAGs are classified as Group V base oil and have suitable properties of high viscosity indices (VI), low NOACK evaporations, and high lubricity. PAGs are categorized into two different types: water-soluble and water-insoluble (newly additional also in oil-soluble) [11]. Oil-soluble PAGs have been becoming more common in the market as they are used for high-temperature applications. PAGs have a high VI and thermal stability. PAGs have greater heat capacities compared to esters and hydrocarbons. The heat capacities of PAGs are around 22% above typical esters and hydrocarbons [12]. The heat capacity of PAGs affects viscosity loss and film-forming abilities. Since PAGs have higher heat capacities, it allows the engine oil to operate at higher temperatures without declining in lubricating performance. Figure 2 shows the heat capacities of alternative oils, such as PAGs, esters, and blends of esters with hydrocarbons (HC-Ester), compared to hydrocarbon-based (HC) oils. Figure 2 displays volumetric heat capacities because an oil pump is defined by a volumetric feed. Esters offer slightly higher volumetric heat capacities surpassed by polyglycols.
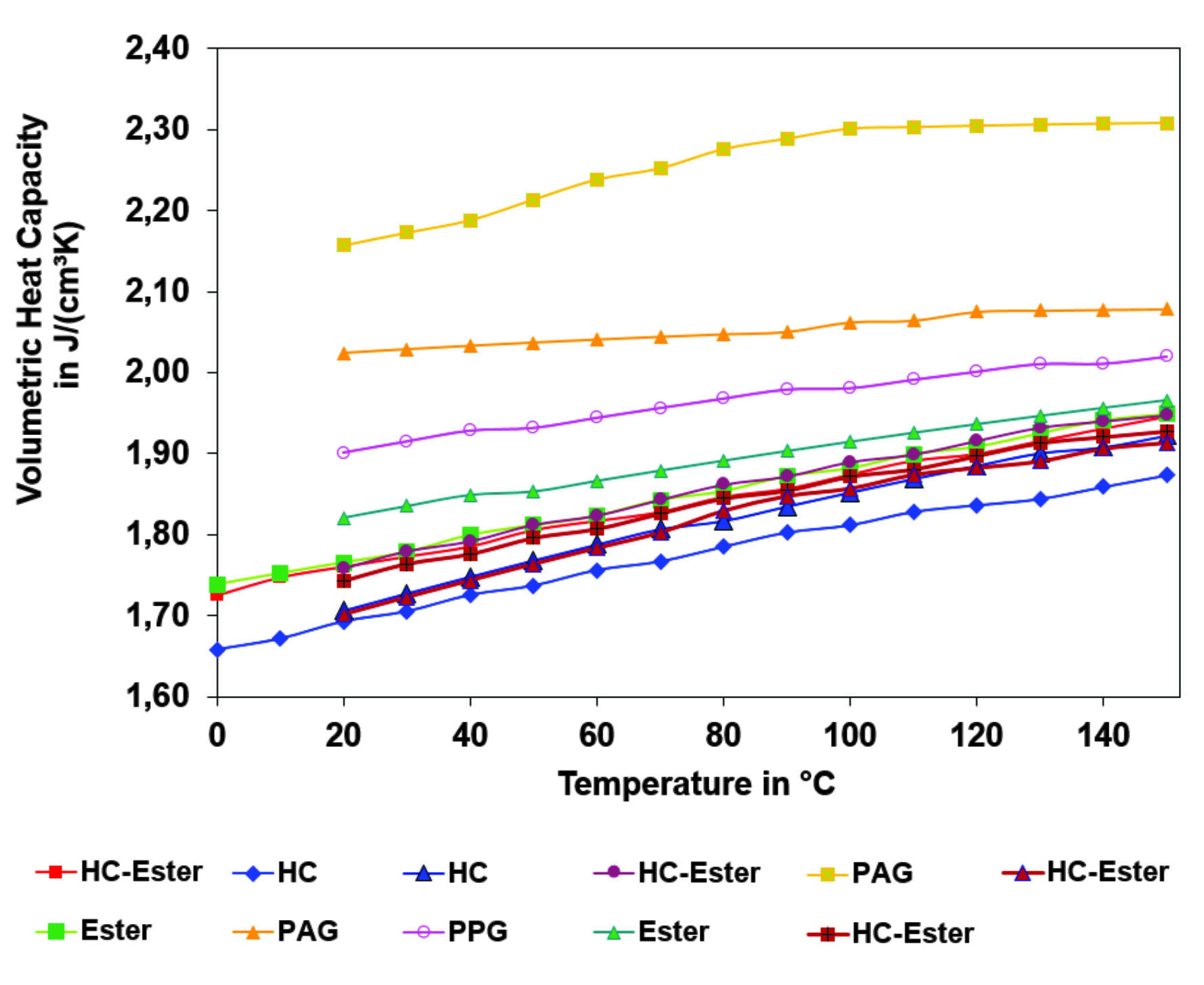
The thermal conductivity of PAGs and esters are slightly higher than hydrocarbons, therefore improving the heat transfer properties of engine oils through the oil film [13]. With high intrinsic VIs, PAG base oils can be used to create low viscosity and low friction engine oils. PAGs also leave little to no residue, making engine oils clean and long-lasting due to their chemical structure [12]. Leaving little to no residue, PAGs are used to reduce friction in engine oils as co-base stock and therefore provide great film strength properties. Low volatility in high temperatures is another property of PAGs, which allows PAG based engine oils to be used in high temperatures as well as low temperatures. PAGs also show low pour points and great oxidative stability. PAGs are used to create low viscosity engine oils by combining them in a formulation that consists of fatty alcohol. When combined with fatty alcohol, the engine oil’s viscosity drops at 40° and VI increases [12, 13]. PAGs have different solvent properties compared to that of hydrocarbons. Since PAGs can be both water and oil-soluble, it allows for more flexibility in engine oil compositions and applications. The water-soluble properties of PAGs allows for easy maintenance of equipment as cleaning the equipment would be easier.
Oil-soluble PAGs (also known as OSPs) are derived from butylene oxides and other kinds of oxides [11, 14] or represents the combination of ethylene oxide/propylene oxide with a fatty alcohol (often C10-C14). OSPs have many beneficial functional properties like that of traditional PAGs. OSPs provide a lot of flexibility in the formulation that affects molecular weights and base stock properties. Table 2 shows the variation that different kinds of OSPs have in different properties, making OSPs versatile in many applications.
| Kin. Viscosity at 40°C [mm2/sec] | D445 | 18 | 32 | 46 | 68 | 150 | 220 | 320 | 460 | 680 |
| Kin. viscosity at 100°C [mm2/sec] | D445 | 4.0 | 6.4 | 8.5 | 11.5 | 23.5 | 33 | 36 | 52 | 77 |
| Viscosity Index | D445 | 123 | 146 | 164 | 166 | 188 | 196 | 163 | 177 | 196 |
| Pour point [°C] | D97 | –41 | –57 | –57 | –53 | –37 | –34 | –37 | –35 | –30 |
| Fire point [°C] | D92 | 220 | 242 | 240 | 258 | 258 | 258 | 260 | 265 | 270 |
| Density at 25°C [g/ml] | D7042 | 0.92 | 0.94 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| Aniline point [°C] | D611 | <–30 | <–30 | <–30 | <–30 | <–30 | –26 | n/d | n/d | n/d |
A unique property of OSPs is their low aniline points of less than –20°. The aniline point is typically used as an indication of determining the solvency or polarity of the base oil. Since the aniline point of OSPs are low, the OSPs are determined to be highly polar. In general, PAGs are moderately polar. This indicates OSPs are more hydrolytically stable compared to esters, bringing a longer lifespan for the engine oil.
Bio-olefins are another type of alternative base oil derived from renewable resources. Olefins are typically processed through crude oil refining and fluid-catalytic cracking, steam cracking, and dehydrogenation. Bio-olefins are olefins that are produced through alternative feedstock, such as biomass [15]. This alternative base oil is garnering more global interest, and technological advances are being researched and designed with new kinds of bio-olefins in mind. Bio-olefins go through processes such as fermentation, gasification, cracking, and deoxygenation. Different processes used to produce bio-olefins create different biomass intermediates. Bio-olefins can be obtained as botryococcene (C30H50) from green algae (botryococcus braunii) or as beta-farnesene (C15H32) from sugar by modified yeast cells. Both are highly branched, and their multiple double bonds need to be eliminated by hydrogenation. From such feedstocks and depending on which kind of process is used, bio alcohols, diols, and other oxygenates are created and used to produce renewable streams for synthetic base oils.
Ethylene is a type of olefin that can be produced through renewable feedstock although it is typically produced through steam cracking from hydrocarbons. Ethylene can be further produced from bio alcohols, plants, and microorganisms [15], depending on which process is being used. The main benefit from creating a base oil of bio-olefins is the content of renewables. Bio-olefins used in base oils can have low volatility, which in turn makes them suited for low viscosity and friction in engine oils.
The next important consideration for ultralow viscosity SAE 4&8 grade oils is the Noack volatility (1h at 250°C) [16]. The physical evaporation (Noack) increases with reducing the viscosity, because at the same time the molar mass of the backbone goes down. The polarities of esters and polyglycols offer here for ultralow viscosity base oils functional benefits of reduced volatilities associated with higher viscosity indices, when compared to hydrocarbons of same kinematic viscosity at 100°C. In summary, several additional requirements and trends create an opportunity frame for alternative base oils.
About the Authors
Raj Shah is a Director at Koehler Instrument Company in New York, where he has worked for the last 25 years. An expert in the field of alternative energy, he is an elected Fellow by his peers at IChemE, STLE, AIC, NLGI, INSTMC, CMI, The Energy Institute, and The Royal Society of Chemistry. A Ph.D in Chemical Engineering from the Pennsylvania state University and a Fellow from the Chartered Management Institute, London, he is also a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute and a Chartered Engineer with the Engineering council, UK. An ASTM Eagle award recipient, Dr. Shah recently coedited the bestseller, “Fuels and Lubricants handbook”. A preview of the handbook (.pdf) is available online.
A 2020 recipient of the illustrious Tau Beta Pi eminent engineer title, he is currently an adjunct professor in the department of Material Science and Chemical engineering at State University of New York, Stony Brook, NY. He has over 250 publications and volunteers on advisory board of directors at several US universities. Read more about Raj in this Penn State alumni spotlight
Dr. Mathias Woydt is managing director of MATRILUB Materials Ι Tribology Ι Lubrication, with more than 34 years of experience in R&D and product development. He has published more than 340 publications and has filed 51 priority patents. He is also board member of the German Society for Tribology. He is an adjunct professor for tribology at the Technical University of Berlin. He is recipient of the ASTM award of Excellence. He can be reached at m.woydt@matrilub.de.
Ms. Hillary Wong is a graduate student at State University of New York (SUNY), Stony brook, and an intern at Koehler Instrument Company.
References
[1] Keegan, Matt, “Valvoline at 150: America’s Oldest Branded Motor Oil, Auto Trends Magazine, May 24, 2016, https://autotrends.org/2016/05/24/valvoline-at-150-americas-oldest-branded-motor-oil
[2] Lee, David, Base oil basics: Quality starts at the base, Chevron Lubricants, February 1, 2018, https://www.chevronlubricants.com/en_us/home/learning/from-chevron/personal-rec-vechicles-and-equipment/base-oil-basics-quality-starts-at-the-base.html
[3] Modern base oils and blending for optimal performance, Lube-Tech, February 2012, pp. 1–7
[4] Base oil groups explained,” Machinery Lubrication, Noria Corporation, October 9, 2012, https://www.machinerylubrication.com/Read/29113/base-oil-groups
[5] “Hydrocracking.” Hydrocracking—an overview, ScienceDirect Topics, 2019, https://www.sciencedirect.com/topics/engineering/hydrocracking
[6] Fitch, Bennett, Understanding the Differences Between Base Oil Formulations, Machinery Lubrication, February 6. 2017, https://www.machinerylubrication.com/Read/30730/base-oil-formulations
[7] Esche, C., et al., Esters for engine oils, Tribology and Lubrication Technology, November 2018, p. 80–82
[8] Esters. BASF AG, Ludwigshafen, https://www.basf.com/global/en/products/segments/industrial_solutions/performance_chemicals/business/fuel-and-lubricant-solutions/esters.html
[9] S. Lucazeau, From jet engine oils to high temperature industrial lubricants, LUBE Magazine 154: 27–29, December 2019
[10] Standard Guide for Selection of Environmentally Acceptable Lubricants for the U.S. Environmental Protection Agency (EPA) Vessel General Permit, ASTM work item WK68688
[11] M.R. Greaves, “Oil Soluble Polyalkylene glycols,” In: Rudnick, L.R. (Ed), Synthetics, Mineral Oils and Biobased Lubricants
[12] Woydt, M., Polyalkylene glycols as next generation engine oils, J. ASTM Int. 8 (6), paper ID JAI103368 and ASTM STP1521, 2012, ISBN: 978-0-8031-7507-5
[13] Schmidt R., G. Klingenberg, and M. Woydt, Thermophysical and viscosimetric properties of environmentally acceptable lubricants, Industrial Lubrication and Tribology 58: 210–224 , 2006, https://doi.org/10.1108/00368790610670809
[14] Greaves, Martin, Oil soluble polyalkylene glycols, LUBE-Tech, Dec. 2013, pp. 1–4.
[15] Zacharopoulou, V. and A.A. Lemonidou, Olefins from biomass intermediates: a review, Catalysts 8(1): 2, 2018, https://doi.org/10.3390/catal8010002
[16] H.R. Henderson, Solving the engine oil puzzle, part 2, Compoundings, February 2018, p. 16–19.



